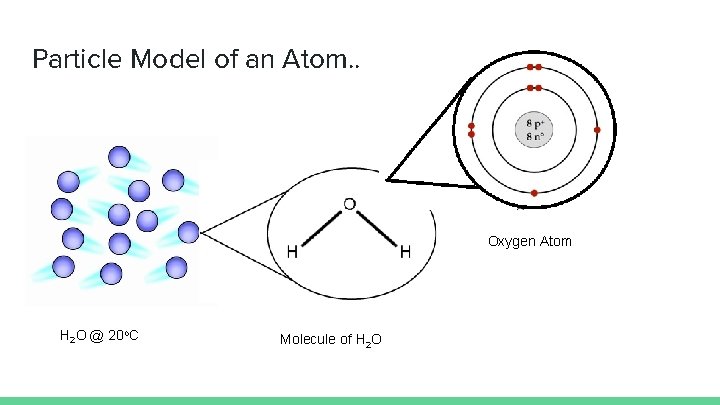
Particle Model of an Atom Oxygen Atom H


- Slides: 16

Particle Model of an Atom. . Oxygen Atom H 2 O @ 20 o. C Molecule of H 2 O

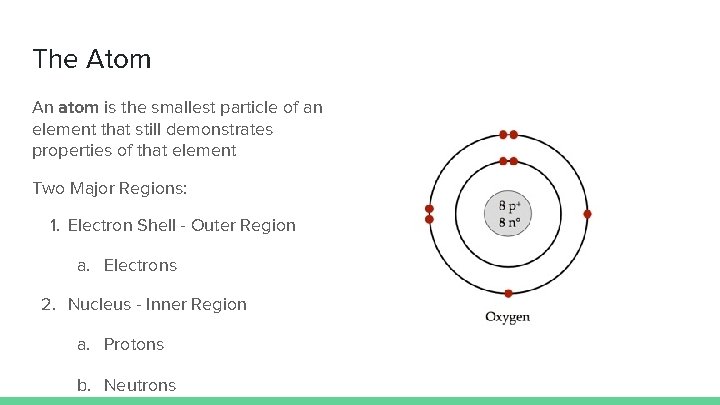
The Atom An atom is the smallest particle of an element that still demonstrates properties of that element Two Major Regions: 1. Electron Shell - Outer Region a. Electrons 2. Nucleus - Inner Region a. Protons b. Neutrons

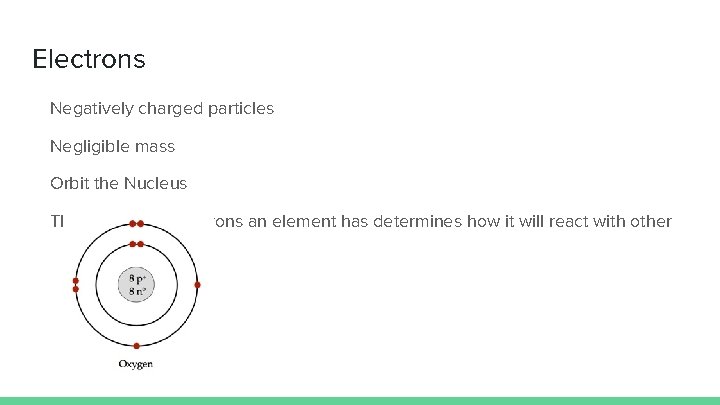
Electrons Negatively charged particles Negligible mass Orbit the Nucleus The number of electrons an element has determines how it will react with other elements

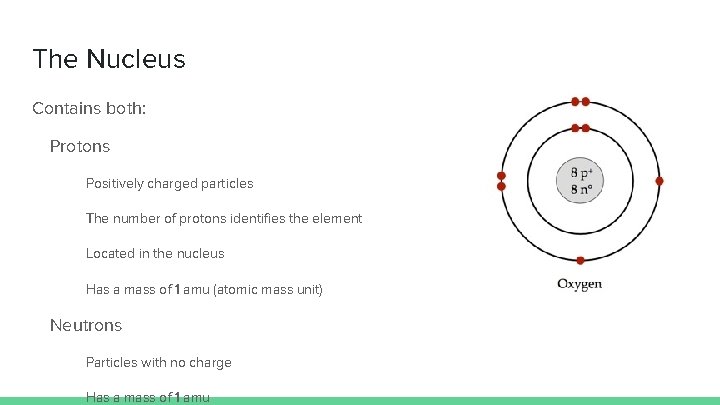
The Nucleus Contains both: Protons Positively charged particles The number of protons identifies the element Located in the nucleus Has a mass of 1 amu (atomic mass unit) Neutrons Particles with no charge Has a mass of 1 amu

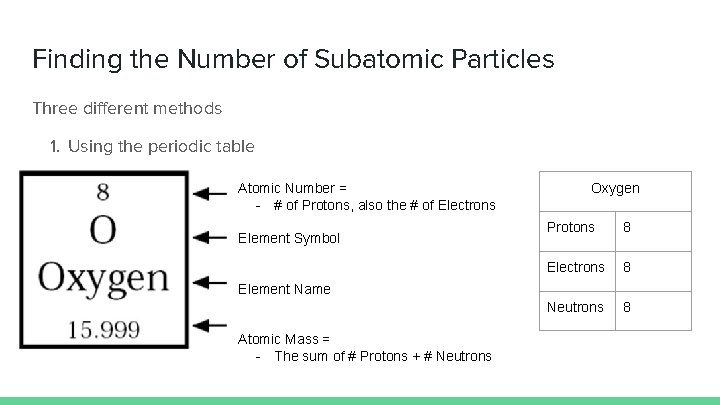
Finding the Number of Subatomic Particles Three different methods 1. Using the periodic table Atomic Number = - # of Protons, also the # of Electrons Element Symbol Oxygen Protons 8 Electrons 8 Neutrons 8 Element Name Atomic Mass = - The sum of # Protons + # Neutrons

Tuesday 12/1 Class Website: dsasse. weebly. com Do Now: Fill in the subatomic particles grid that we started yesterday in class. Find the protons, neutrons, and electrons of each of element. I will be able to… Perform weighted-average calculations Use a periodic table to determine the number of subatomic particles in an element Use models to represent isotopes HW: 1. Isotope Calculations & Grid WS 2. Hand Warmer Report due to Google Classroom by tonight at 11: 59 pm with a homework extension.

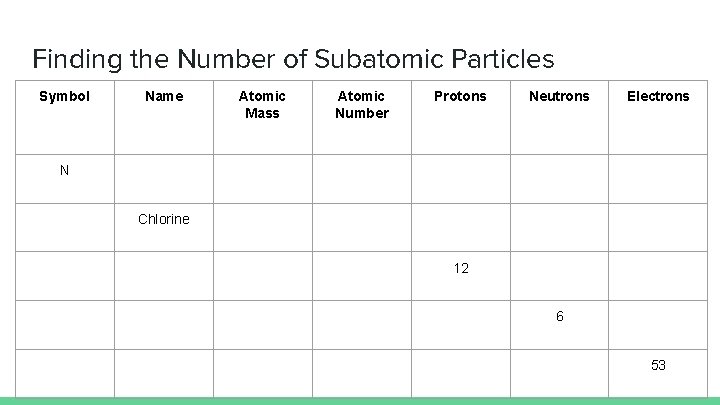
Finding the Number of Subatomic Particles Symbol Name Atomic Mass Atomic Number Protons Neutrons Electrons N Chlorine 12 6 53

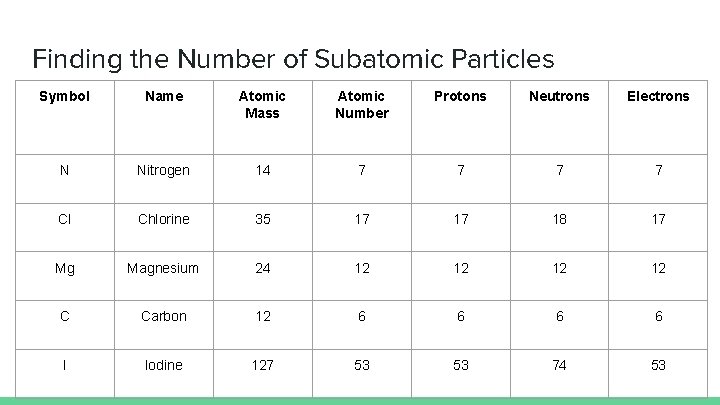
Finding the Number of Subatomic Particles Symbol Name Atomic Mass Atomic Number Protons Neutrons Electrons N Nitrogen 14 7 7 Cl Chlorine 35 17 17 18 17 Mg Magnesium 24 12 12 C Carbon 12 6 6 I Iodine 127 53 53 74 53

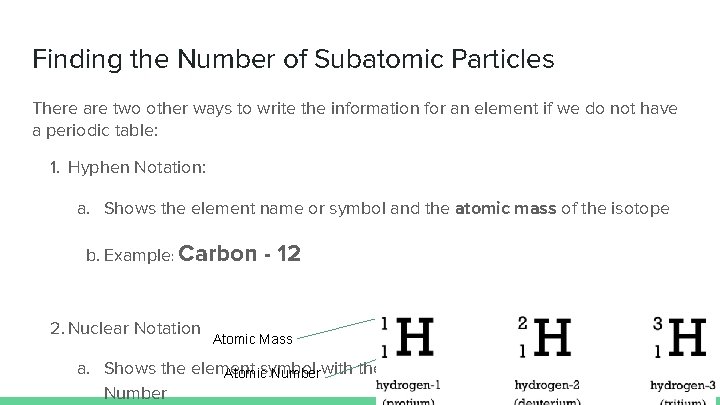
Finding the Number of Subatomic Particles There are two other ways to write the information for an element if we do not have a periodic table: 1. Hyphen Notation: a. Shows the element name or symbol and the atomic mass of the isotope b. Example: Carbon 2. Nuclear Notation - 12 Atomic Mass a. Shows the element symbol Atomic Numberwith the Atomic Mass above the Atomic Number

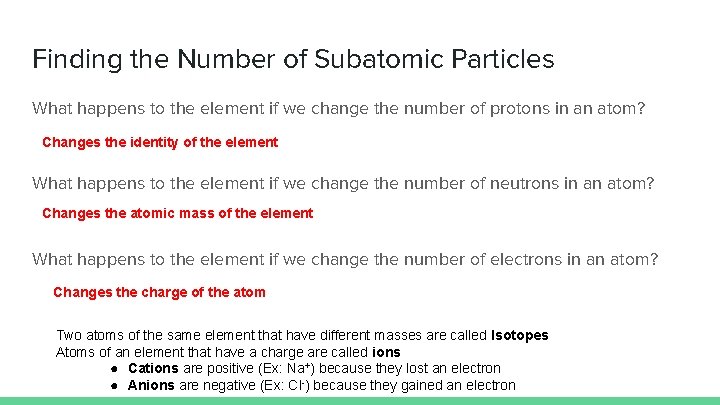
Finding the Number of Subatomic Particles What happens to the element if we change the number of protons in an atom? Changes the identity of the element What happens to the element if we change the number of neutrons in an atom? Changes the atomic mass of the element What happens to the element if we change the number of electrons in an atom? Changes the charge of the atom Two atoms of the same element that have different masses are called Isotopes Atoms of an element that have a charge are called ions ● Cations are positive (Ex: Na+) because they lost an electron ● Anions are negative (Ex: Cl-) because they gained an electron

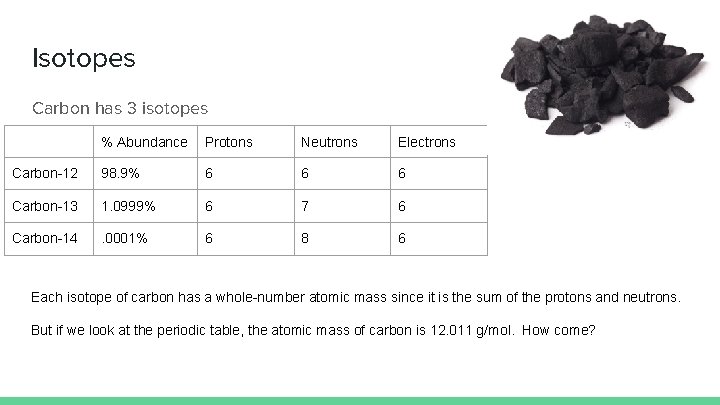
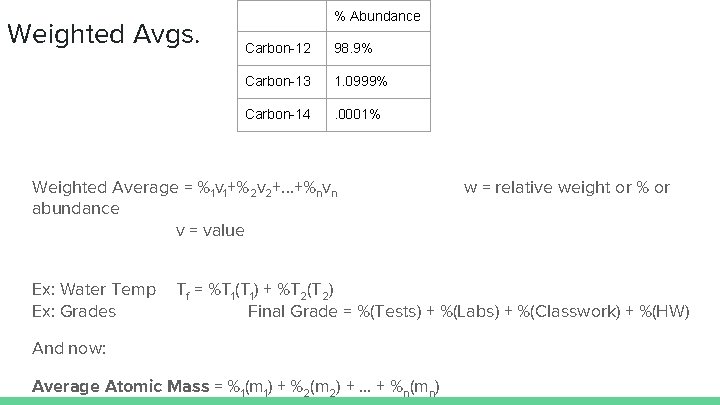
Isotopes Carbon has 3 isotopes % Abundance Protons Neutrons Electrons Carbon-12 98. 9% 6 6 6 Carbon-13 1. 0999% 6 7 6 Carbon-14 . 0001% 6 8 6 Each isotope of carbon has a whole-number atomic mass since it is the sum of the protons and neutrons. But if we look at the periodic table, the atomic mass of carbon is 12. 011 g/mol. How come?

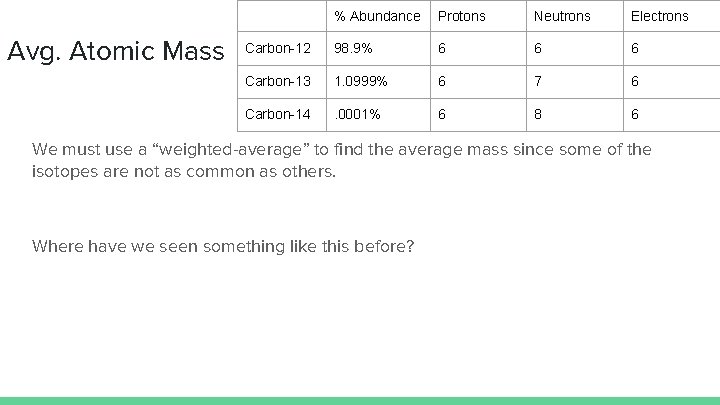
Avg. Atomic Mass % Abundance Protons Neutrons Electrons Carbon-12 98. 9% 6 6 6 Carbon-13 1. 0999% 6 7 6 Carbon-14 . 0001% 6 8 6 We must use a “weighted-average” to find the average mass since some of the isotopes are not as common as others. Where have we seen something like this before?

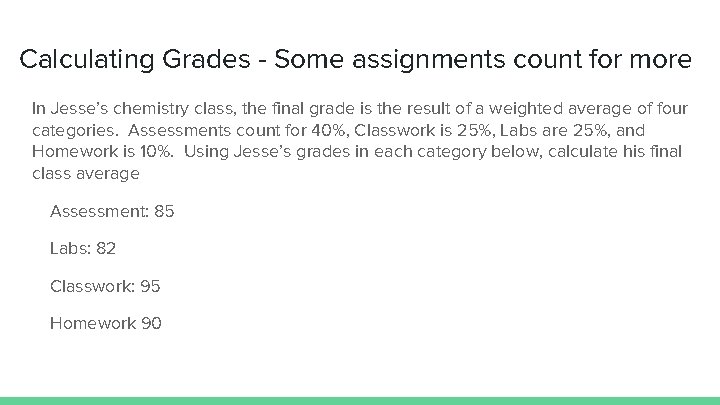
Calculating Grades - Some assignments count for more In Jesse’s chemistry class, the final grade is the result of a weighted average of four categories. Assessments count for 40%, Classwork is 25%, Labs are 25%, and Homework is 10%. Using Jesse’s grades in each category below, calculate his final class average Assessment: 85 Labs: 82 Classwork: 95 Homework 90

Final Temp of mixing water We were calculating what percent of the total water was hot/cold

Weighted Avgs. % Abundance Carbon-12 98. 9% Carbon-13 1. 0999% Carbon-14 . 0001% Weighted Average = %1 v 1+%2 v 2+. . . +%nvn abundance v = value Ex: Water Temp Ex: Grades w = relative weight or % or Tf = %T 1(T 1) + %T 2(T 2) Final Grade = %(Tests) + %(Labs) + %(Classwork) + %(HW) And now: Average Atomic Mass = %1(m 1) + %2(m 2) + … + %n(mn)

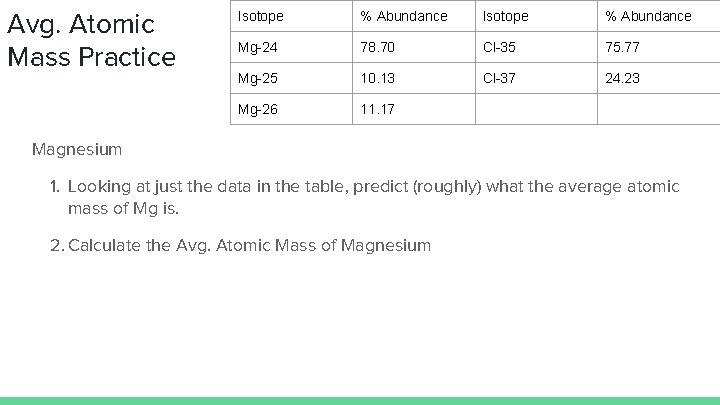
Avg. Atomic Mass Practice Isotope % Abundance Mg-24 78. 70 Cl-35 75. 77 Mg-25 10. 13 Cl-37 24. 23 Mg-26 11. 17 Magnesium 1. Looking at just the data in the table, predict (roughly) what the average atomic mass of Mg is. 2. Calculate the Avg. Atomic Mass of Magnesium