Partial Molar Quantities Activities Mixing Properties Composition X







- Slides: 28

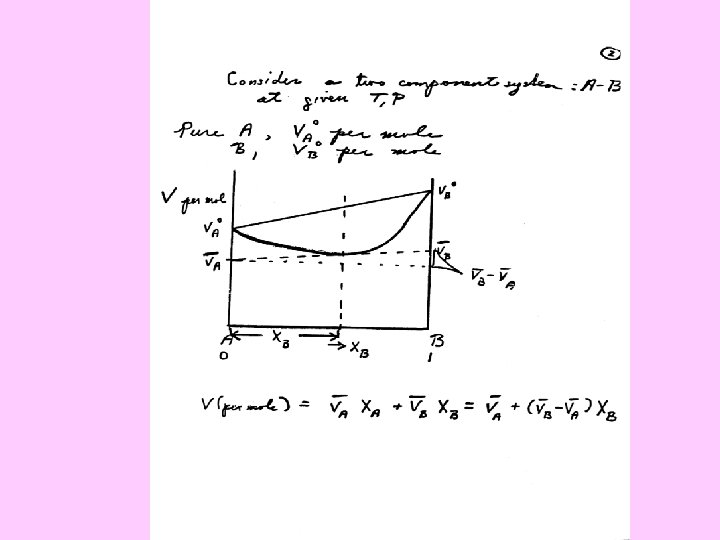
Partial Molar Quantities, Activities, Mixing Properties Composition (X) is a critical variable, as well at temperature (T) and pressure (P) Variation of a thermodynamic parameter with number of moles of one component, all other compositional variables, T, P held constant



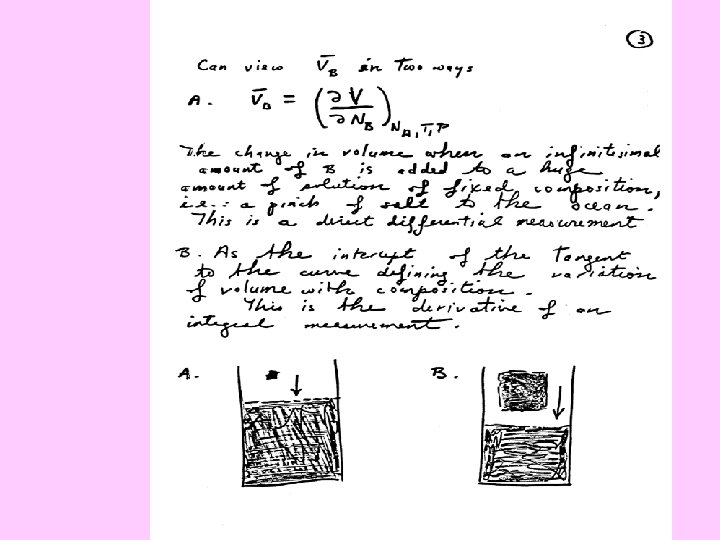
Partial Molar Volume

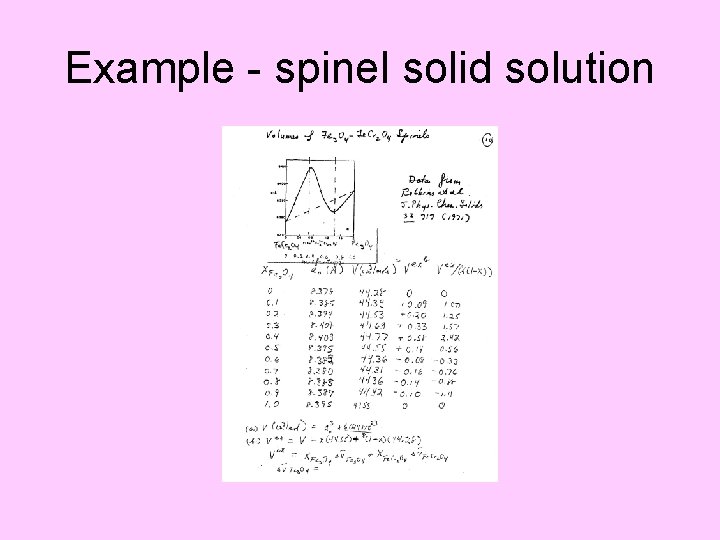
Example - spinel solid solution

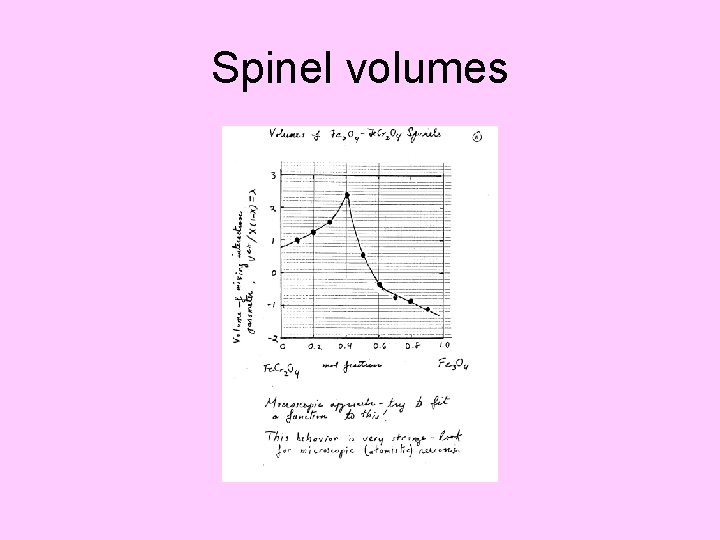
Spinel volumes



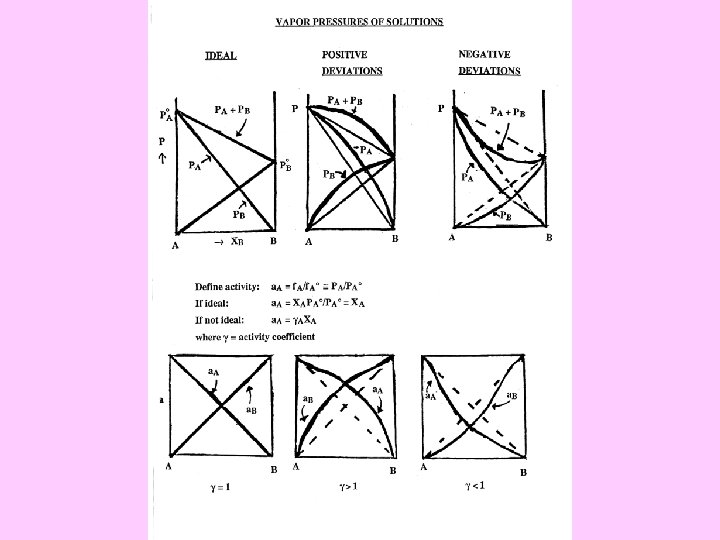
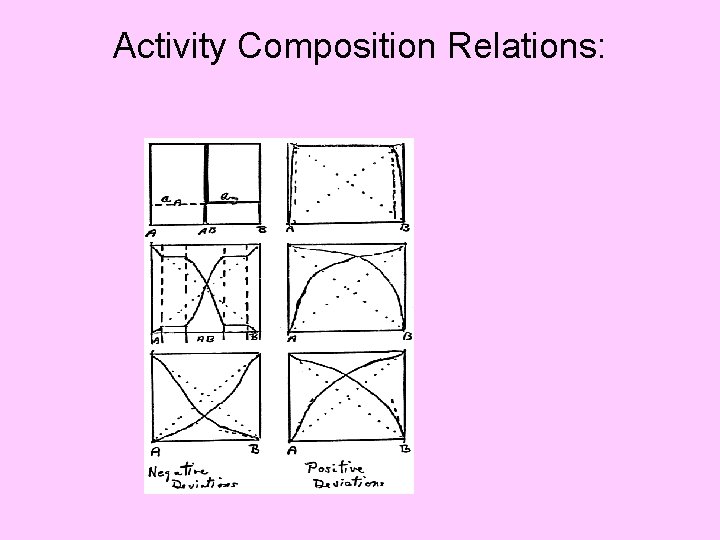
Activity Composition Relations:

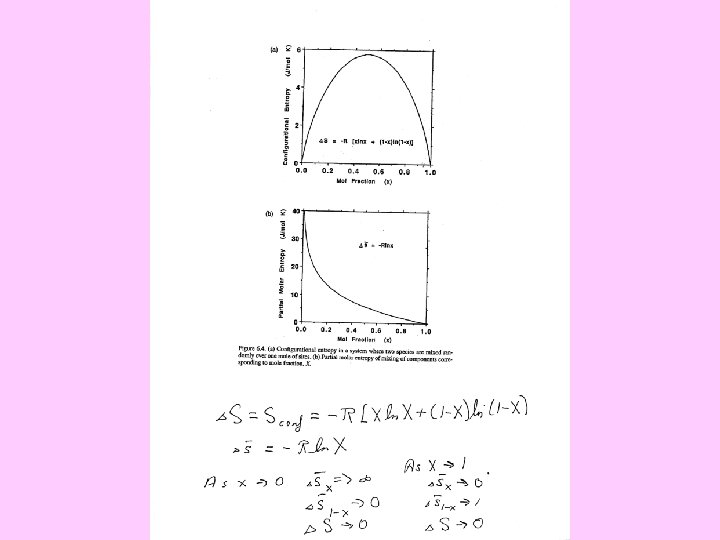
The Entropy of Mixing in Solid Solutions Contributions vibrational magnetic and electronic configurational For the random mixing of a total of one mole of species over a total of one mole of sites, ∆Smix = -R[x. A ln x. A + x. B ln x. B] and ∆s¯(A) = -R ln x. A , ∆s¯(B) = -R ln x. B


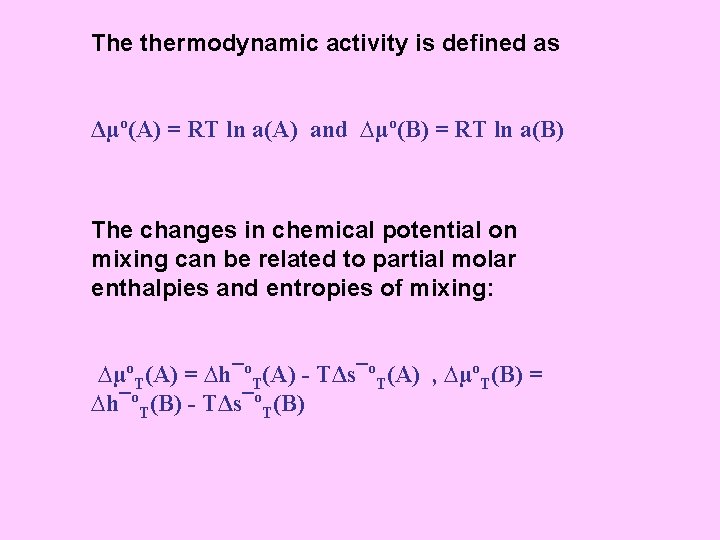
The thermodynamic activity is defined as Δµº(A) = RT ln a(A) and ∆µº(B) = RT ln a(B) The changes in chemical potential on mixing can be related to partial molar enthalpies and entropies of mixing: ∆µºT(A) = ∆h¯ºT(A) - TΔs¯ºT(A) , ∆µºT(B) = ∆h¯ºT(B) - TΔs¯ºT(B)

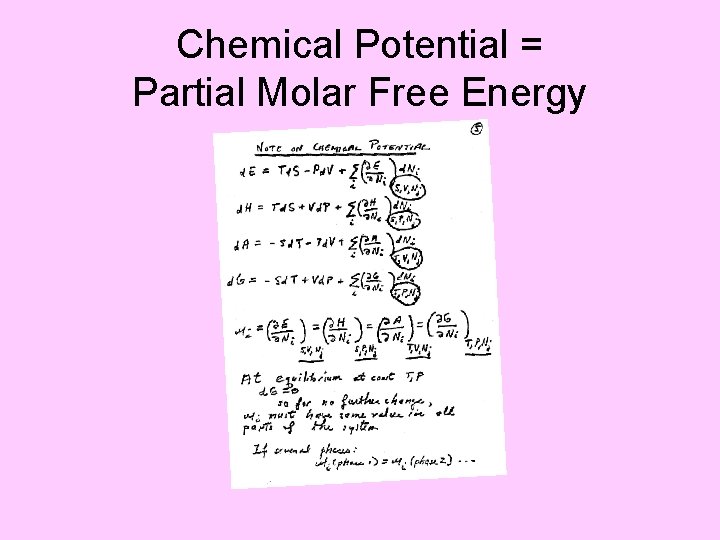
Chemical Potential = Partial Molar Free Energy

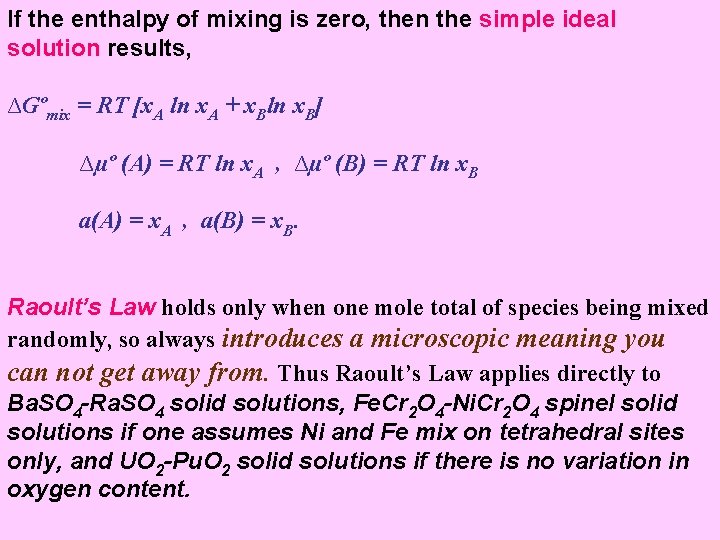
If the enthalpy of mixing is zero, then the simple ideal solution results, ∆Gºmix = RT [x. A ln x. A + x. Bln x. B] ∆µº (A) = RT ln x. A , ∆µº (B) = RT ln x. B a(A) = x. A , a(B) = x. B. Raoult’s Law holds only when one mole total of species being mixed randomly, so always introduces a microscopic meaning you can not get away from. Thus Raoult’s Law applies directly to Ba. SO 4 -Ra. SO 4 solid solutions, Fe. Cr 2 O 4 -Ni. Cr 2 O 4 spinel solid solutions if one assumes Ni and Fe mix on tetrahedral sites only, and UO 2 -Pu. O 2 solid solutions if there is no variation in oxygen content.

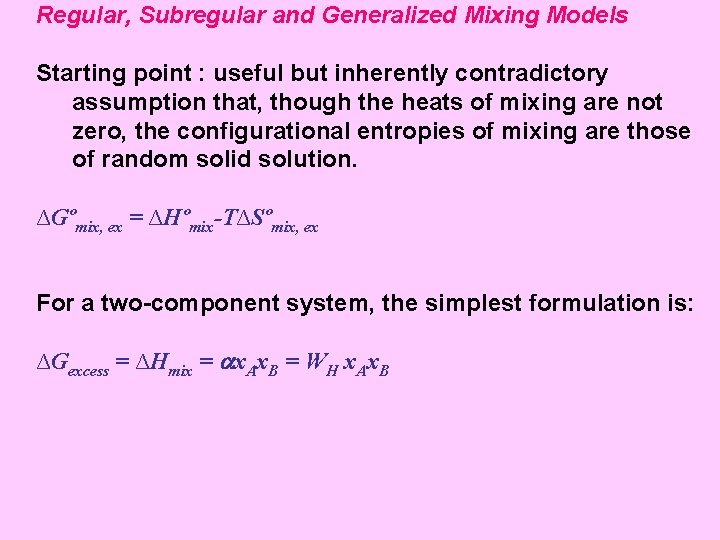
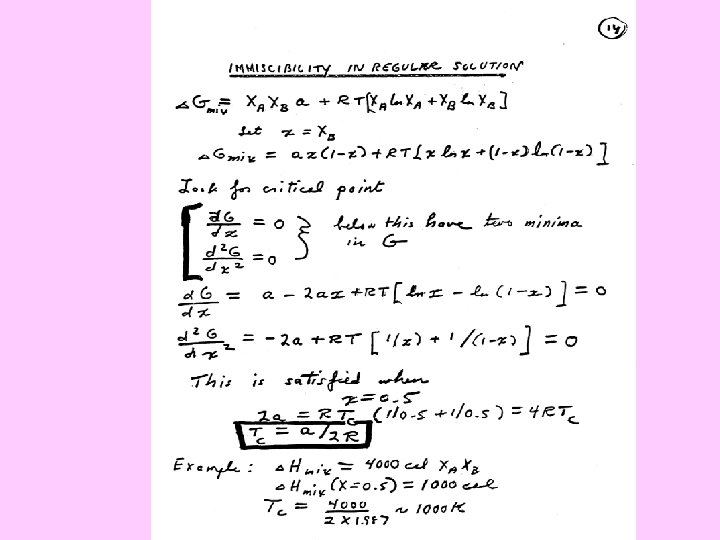
Regular, Subregular and Generalized Mixing Models Starting point : useful but inherently contradictory assumption that, though the heats of mixing are not zero, the configurational entropies of mixing are those of random solid solution. ∆Gºmix, ex = ∆Hºmix-T∆Sºmix, ex For a two-component system, the simplest formulation is: ∆Gexcess = ∆Hmix = x. Ax. B = WH x. Ax. B

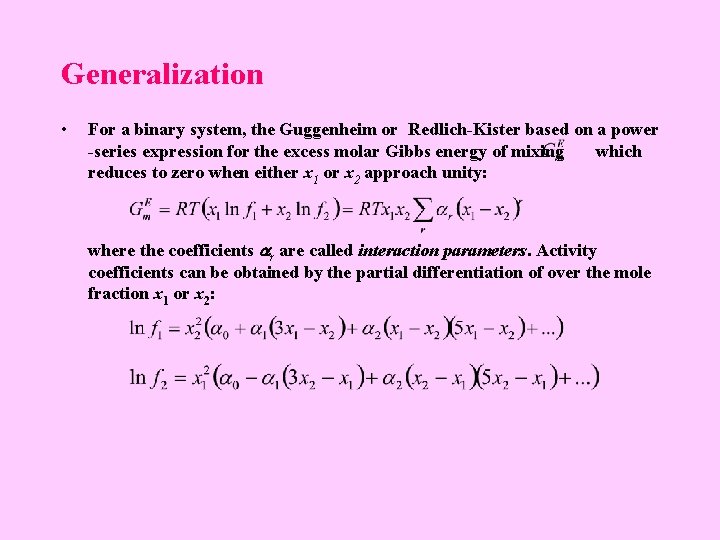
Generalization • For a binary system, the Guggenheim or Redlich-Kister based on a power -series expression for the excess molar Gibbs energy of mixing which reduces to zero when either x 1 or x 2 approach unity: where the coefficients r are called interaction parameters. Activity coefficients can be obtained by the partial differentiation of over the mole fraction x 1 or x 2:

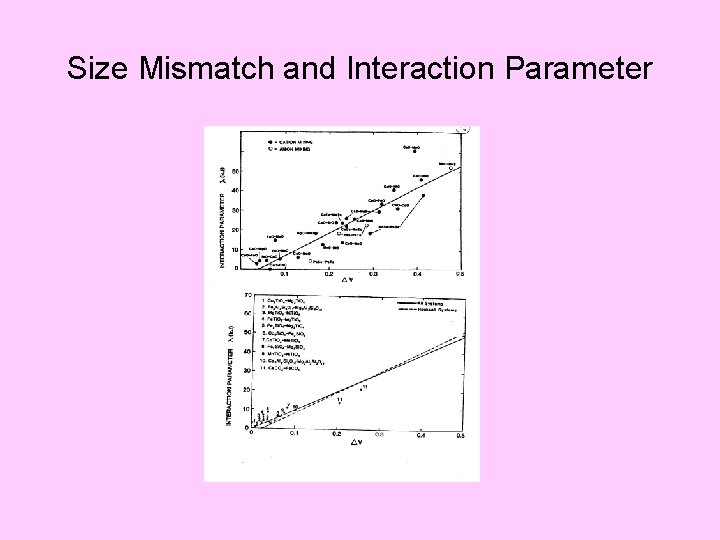
Systematics in Mixing Propertieszz; (Davies and Navrotsky 1981)

Size Mismatch and Interaction Parameter

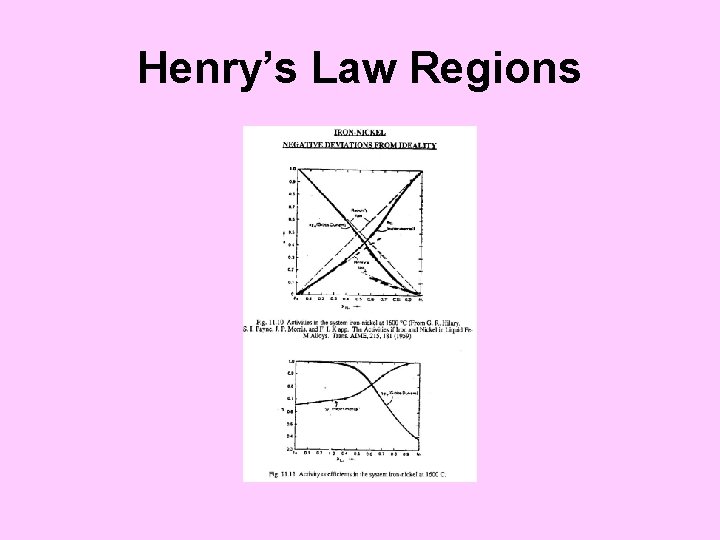
Henry’s Law Regions

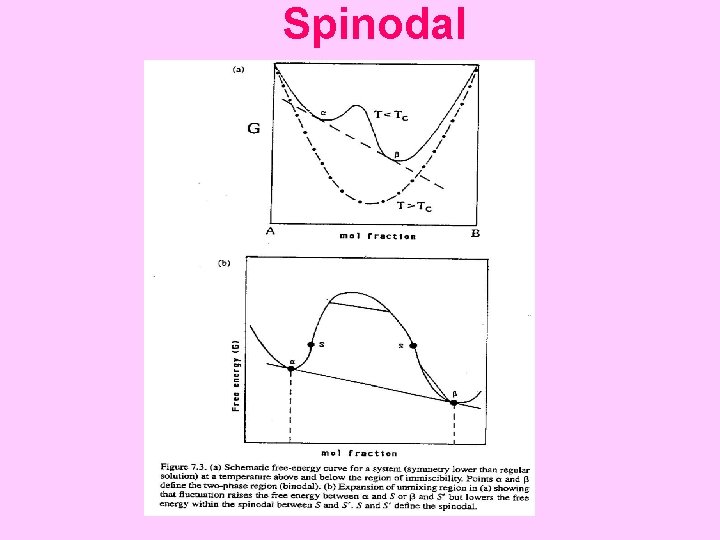
IMMISCIBILITY Immiscibility (phase separation) occurs when positive WG terms outweigh the configurational entropy contribution. For the strictly regular solution, the miscibility gap closes at a critical point or consolute temperature. T = WH/2 R Conditions for equilibrium between two phases (α and β) : simultaneous equalities of chemical potential or activities: µ(A, phase α) = µ(A, phase β) µ(B, phase α) = µ(B, phase β) a(A, phase α) = a(A, phase β) a(B, phase α) = a(B, phase β)



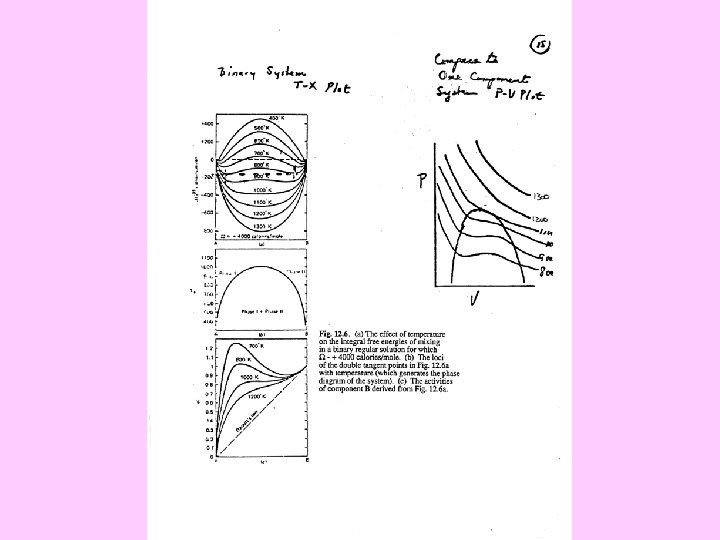
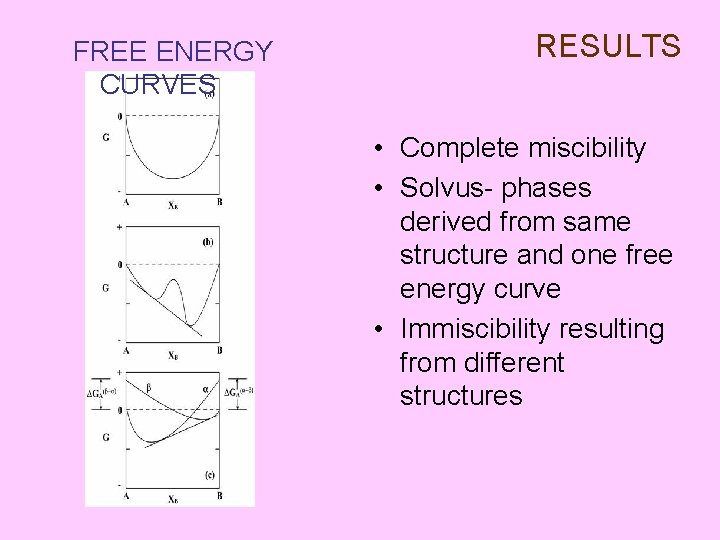
FREE ENERGY CURVES RESULTS • Complete miscibility • Solvus- phases derived from same structure and one free energy curve • Immiscibility resulting from different structures

Phases with Different Structures Partial solid solution can exist among end members of different structure: A with structure “α” and B with structure “β”. µ(A, α) = µº(A, α) + RT ln a(A, α) µ(A, β) = µº(A, α) + ∆µ(A, α→β) + RT ln a(A, β) µ(B, α) = µº(B, β) + ∆µ(B, β→α) + RT ln a(B, α) µ(B, β) = µº(B, β) + RT ln a(B, β) The limiting solubilities are given by equating chemical potentials: µ(A, α) = µ(A, β) µ(B, α) = µ(B, β) The miscibility gap can not close and is not

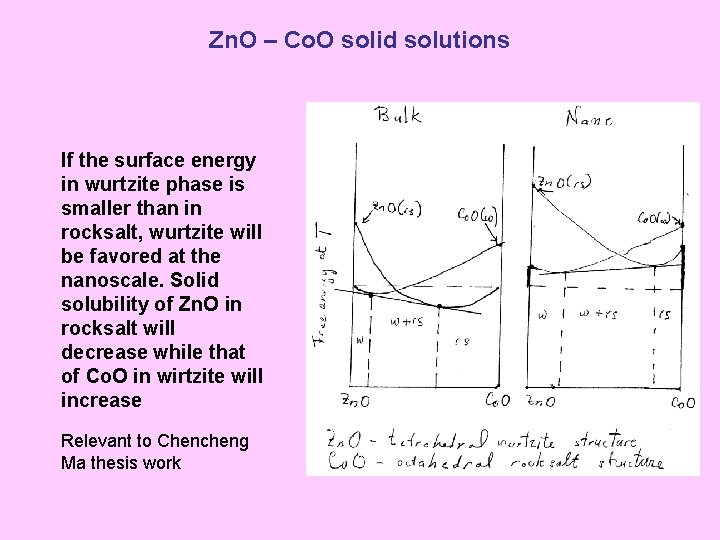
Zn. O – Co. O solid solutions If the surface energy in wurtzite phase is smaller than in rocksalt, wurtzite will be favored at the nanoscale. Solid solubility of Zn. O in rocksalt will decrease while that of Co. O in wirtzite will increase Relevant to Chencheng Ma thesis work

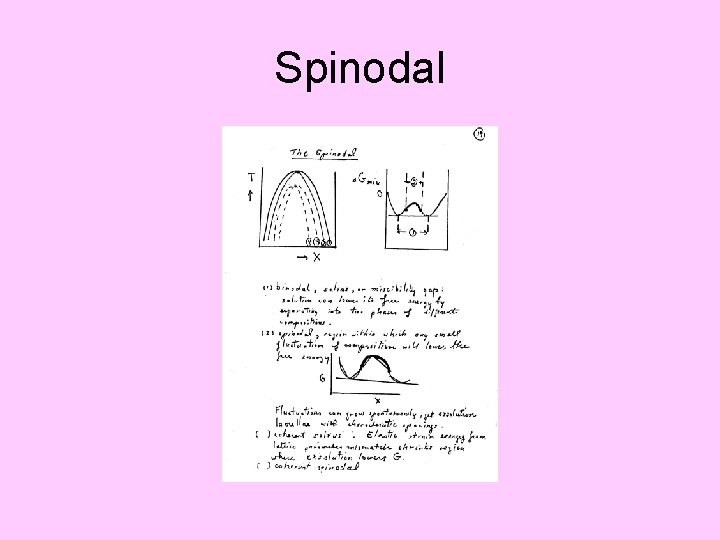
Spinodal

Spinodal

References • • • Guggenheim, E. A. , Thermodynamics: An advanced treatment for chemists and physicists. 5 th edn. Amsterdam: North-Holland; 390, 1967 Thompson, J. B. , Thermodynamic properties of simple solutions. In Researches in geochemistry. Edited by Abelson PH. New York: John Wiley and Sons; 1967 340 -361. Thompson, J. B. , Chemical reactions in crystals. Amer. Mineral. , 54 (1969) 341 -375. Eriksson, G. , Rosen, E. , Thermodynamic studies of high temperature equilibria. VIII: General equations for the calculation of equilibria in multiphase systems. Chemica Scripta, 4(4) (1973) 193 -194. Pelton, A. D. , Bale, C. W. , Computational techniques for the treatment of thermodinamic data in multicomponent systems and the calculation of phase equilibria. Calphad, 1(3) (1977) 253 -273. Wood, B. J. , Nicholls, J. , The thermodynamic properties of reciprocal solid solutions. Contributions to Mineralogy and Petrology 66 (1978) 389 -400. Nordstrom, D. K. , Munoz, J. L. , Geochemical thermodynamics. 2 nd edn. Boston: Blackwell Scientific Publications (1994) 483. Ott, J. B. , Boerio-Goates, J. , Chemical thermodynamics: Advanced applications. San Diego CA: Academic Press, 438 (2000). Ott, J. B. , Boerio-Goates J. , Chemical thermodynamics: Principles and applications. San Diego CA: Academic Press, 664 (2000). Ganguly, J. , Thermodynamic modelling of solid solutions. In EMU Notes in Mineralogy: Solid solutions in silicate and oxide systems of geological importance. Edited by Geiger CA. Budapest: Eotvos University Press, 3 (2001) 37 -69. Geiger, C. A. , Solid solutions in silicate and oxide systems of geological importance. In European mineralogical union notes in mineralogy. Edited by Papp G, Weiszburg TG. Budapest: Eotvos University Press, 3 (2001) 458.