Part III Heat Chapter 21 Temperature Heat Thermal





- Slides: 17

Part III: Heat Chapter 21 Temperature, Heat & Thermal Expansion 07 -Oct-20 Physics 1 (Garcia) SJSU

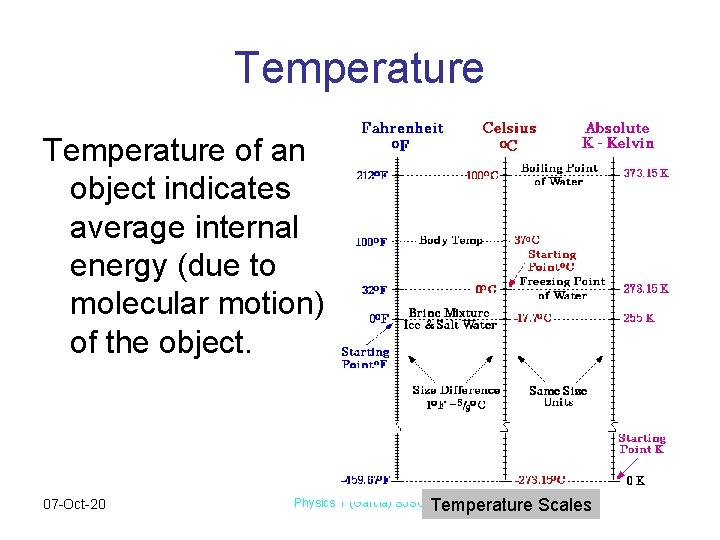
Temperature of an object indicates average internal energy (due to molecular motion) of the object. 07 -Oct-20 Physics 1 (Garcia) SJSU Temperature Scales

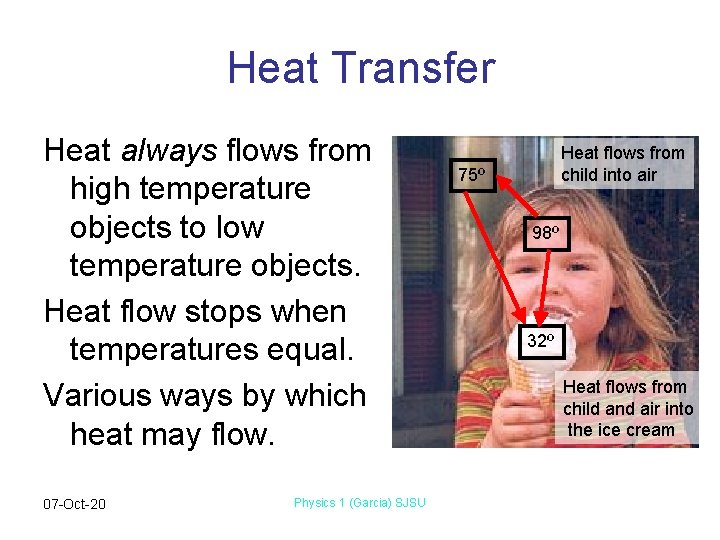
Heat Transfer Heat always flows from high temperature objects to low temperature objects. Heat flow stops when temperatures equal. Various ways by which heat may flow. 07 -Oct-20 Physics 1 (Garcia) SJSU Heat flows from child into air 75º 98º 32º Heat flows from child and air into the ice cream

Temperature Conversions To convert between Celsius and Fahrenheit temperatures, use the following equation • F=9/5 C + 32 • C=(F-32)*5/9 To Convert between Celsius and Kelvin temperatures, use the following K=C+273 C=K-273 07 -Oct-20 Physics 1 (Garcia) SJSU

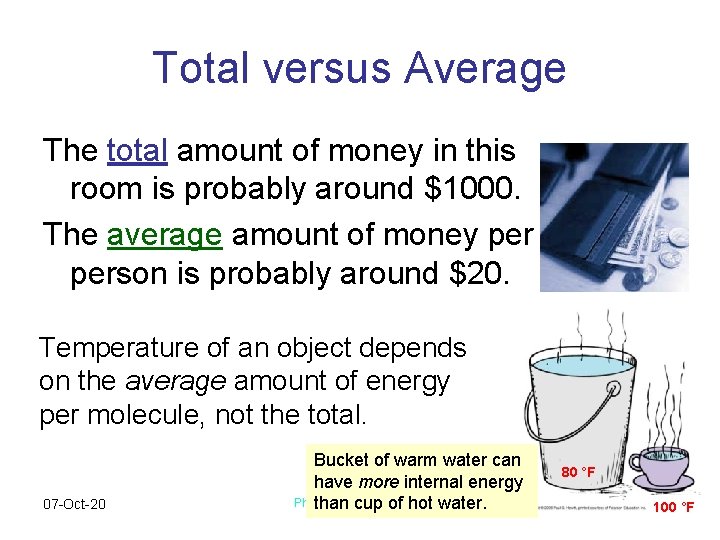
Total versus Average The total amount of money in this room is probably around $1000. The average amount of money person is probably around $20. Temperature of an object depends on the average amount of energy per molecule, not the total. 07 -Oct-20 Bucket of warm water can have more internal energy Physics 1 (Garcia) than cup of. SJSU hot water. 80 °F 100 °F

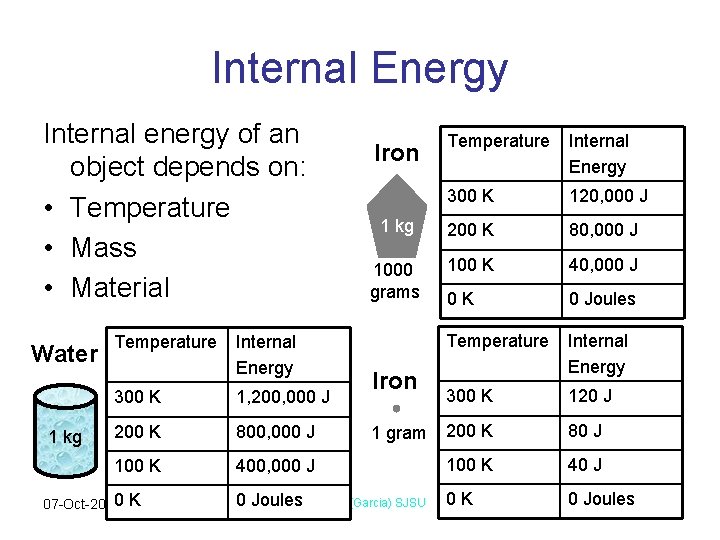
Internal Energy Internal energy of an object depends on: • Temperature • Mass • Material Water 1 kg 07 -Oct-20 Temperature Internal Energy 300 K 120, 000 J 1 kg 200 K 80, 000 J 1000 grams 100 K 40, 000 J 0 K 0 Joules Temperature Internal Energy 300 K 120 J 200 K 80 J Iron Temperature Internal Energy 300 K 1, 200, 000 J 200 K 800, 000 J 100 K 40 J 0 K 0 Joules. Physics 1 (Garcia) SJSU 0 K 0 Joules Iron 1 gram

Money and Happiness Some people need a lot of money to make them happy. Some don’t. Some materials, such as water, need a lot of energy to raise their temperature. Some materials, such as iron, need little energy to raise their temperature. 07 -Oct-20 Physics 1 (Garcia) SJSU Nicole Richie & Paris Hilton MAHATMA GANDHI

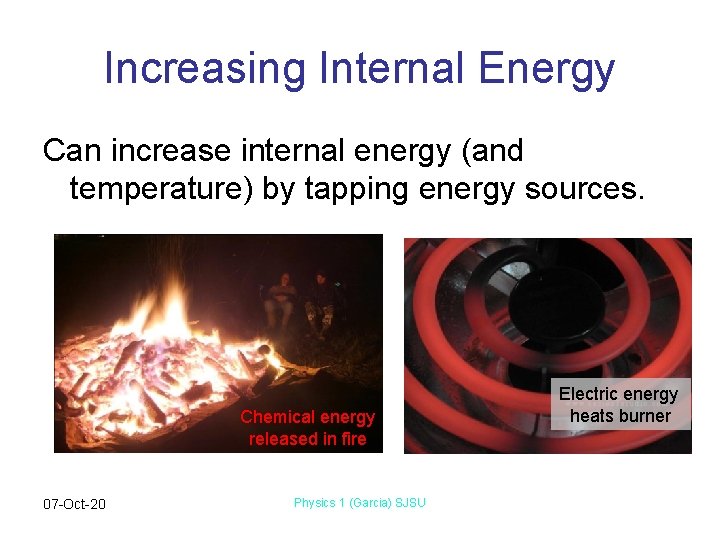
Increasing Internal Energy Can increase internal energy (and temperature) by tapping energy sources. Chemical energy released in fire 07 -Oct-20 Physics 1 (Garcia) SJSU Electric energy heats burner

Work and Heat May increase internal energy by exerting a force to do mechanical work. Rub hands together for warmth 07 -Oct-20 Physics 1 (Garcia) SJSU Strike an iron surface with great force and red-hot sparks are created

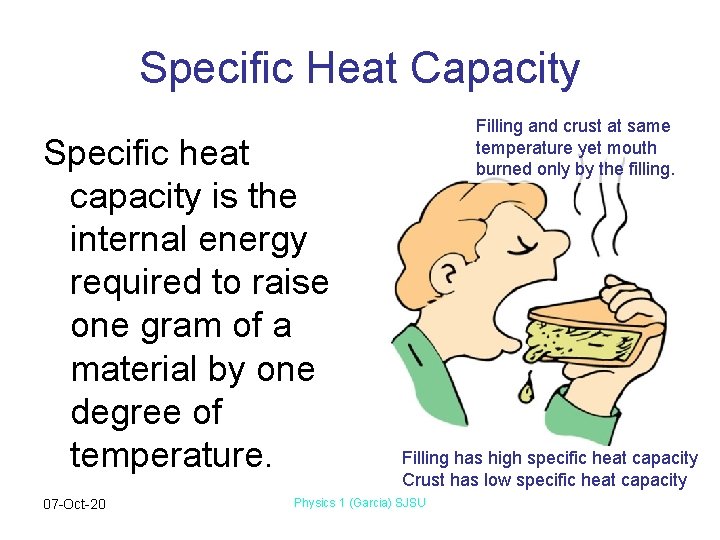
Specific Heat Capacity Specific heat capacity is the internal energy required to raise one gram of a material by one degree of temperature. 07 -Oct-20 Filling and crust at same temperature yet mouth burned only by the filling. Filling has high specific heat capacity Crust has low specific heat capacity Physics 1 (Garcia) SJSU

Check Yourself Why does a piece of watermelon stay cool for a longer time than sandwiches do when both are removed from a cooler on a hot day? Why is it that the climate in the desert is so hot during the day yet so cold at night? 07 -Oct-20 Physics 1 (Garcia) SJSU

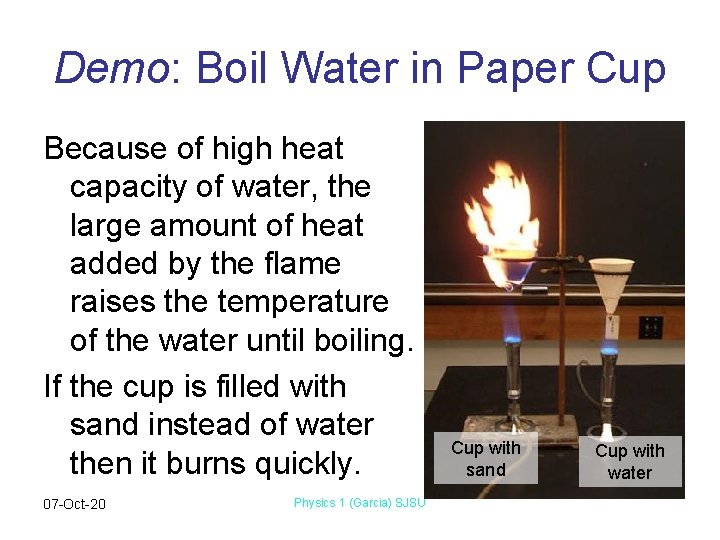
Demo: Boil Water in Paper Cup Because of high heat capacity of water, the large amount of heat added by the flame raises the temperature of the water until boiling. If the cup is filled with sand instead of water then it burns quickly. 07 -Oct-20 Physics 1 (Garcia) SJSU Cup with sand Cup with water

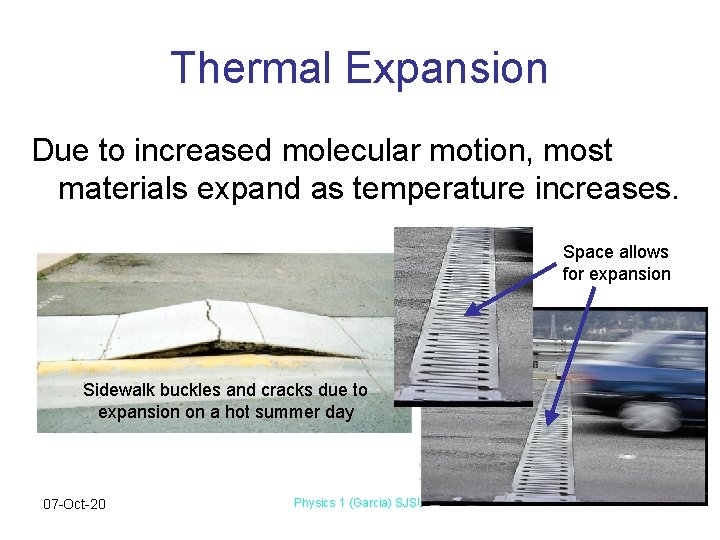
Thermal Expansion Due to increased molecular motion, most materials expand as temperature increases. Space allows for expansion Sidewalk buckles and cracks due to expansion on a hot summer day 07 -Oct-20 Physics 1 (Garcia) SJSU

Thermal Expansion • Water is an exception. – Water contracts as it cools down to 4 degrees Celsius, then expands as it cools down to 0 degrees Celsius. • This is how pot holes form, soda bottles explode when left in the freezer, and ice cubes float 07 -Oct-20 Physics 1 (Garcia) SJSU

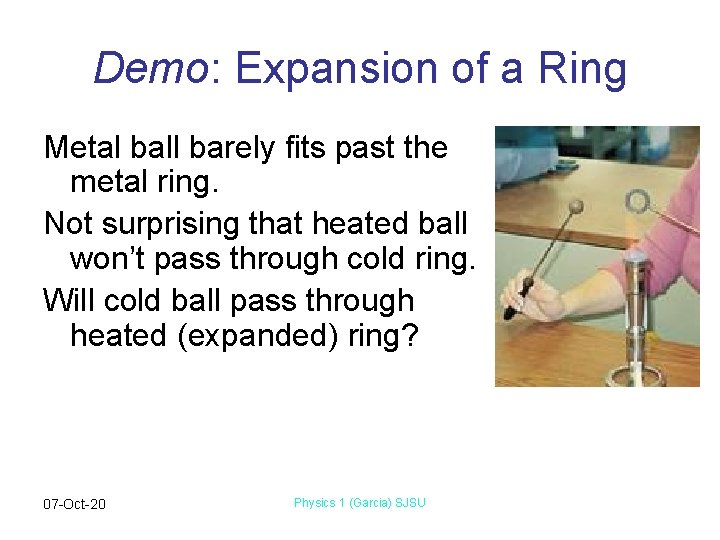
Demo: Expansion of a Ring Metal ball barely fits past the metal ring. Not surprising that heated ball won’t pass through cold ring. Will cold ball pass through heated (expanded) ring? 07 -Oct-20 Physics 1 (Garcia) SJSU

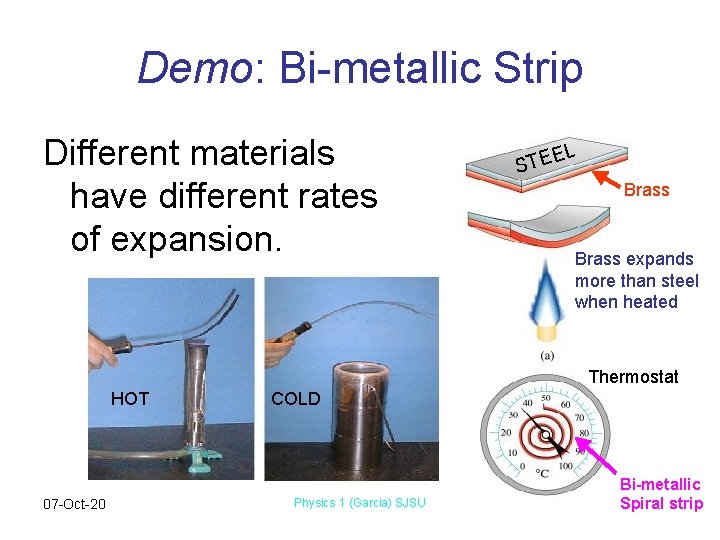
Demo: Bi-metallic Strip Different materials have different rates of expansion. L E STE Brass expands more than steel when heated Thermostat HOT 07 -Oct-20 COLD Physics 1 (Garcia) SJSU Bi-metallic Spiral strip

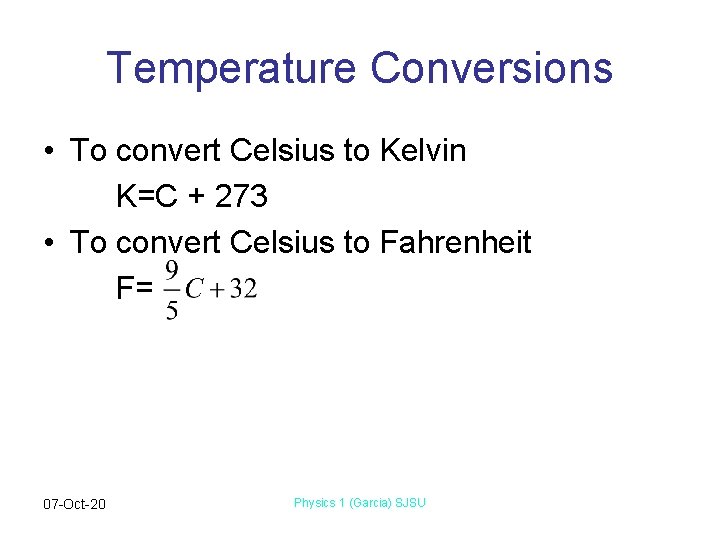
Temperature Conversions • To convert Celsius to Kelvin K=C + 273 • To convert Celsius to Fahrenheit F= 07 -Oct-20 Physics 1 (Garcia) SJSU