Part 6 Isocratic vs gradient eluent conditions comparison
- Slides: 5

Part 6 - Isocratic vs gradient eluent conditions comparison Prof. Dr Snežana Maletić, Prof. Dr Ivana Ivančev Tumbas Technical assistance: Dr Malcolm Watson, Dr Anita Leovac Maćerak Course: Environmental Quality Control (Advanced Course) – lab exercise University of Novi Sad, Faculty of Sciences, Trg Dositeja Obradovića 3, 21000 Novi Sad, Republic of Serbia _____________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

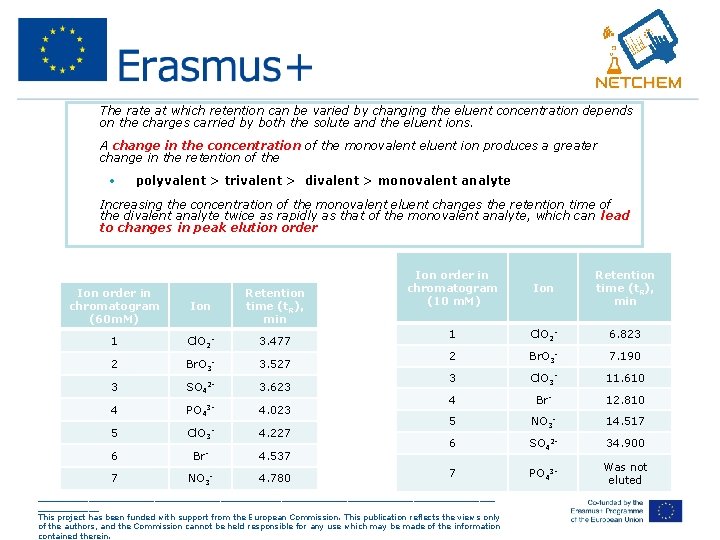
• The rate at which retention can be varied by changing the eluent concentration depends on the charges carried by both the solute and the eluent ions. • A change in the concentration of the monovalent eluent ion produces a greater change in the retention of the • • polyvalent > trivalent > divalent > monovalent analyte Increasing the concentration of the monovalent eluent changes the retention time of the divalent analyte twice as rapidly as that of the monovalent analyte, which can lead to changes in peak elution order Ion order in chromatogram (60 m. M) Ion Retention time (t. R), min 1 Cl. O 2 - 3. 477 2 Br. O 3 - 3. 527 3 SO 42 - 3. 623 4 PO 43 - 4. 023 5 Cl. O 3 4. 227 6 Br- 4. 537 7 NO 3 - 4. 780 - Ion order in chromatogram (10 m. M) Ion Retention time (t. R), min 1 Cl. O 2 - 6. 823 2 Br. O 3 - 7. 190 3 Cl. O 3 - 11. 610 4 Br- 12. 810 5 NO 3 - 14. 517 6 SO 42 - 34. 900 7 PO 43 - Was not eluted _____________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

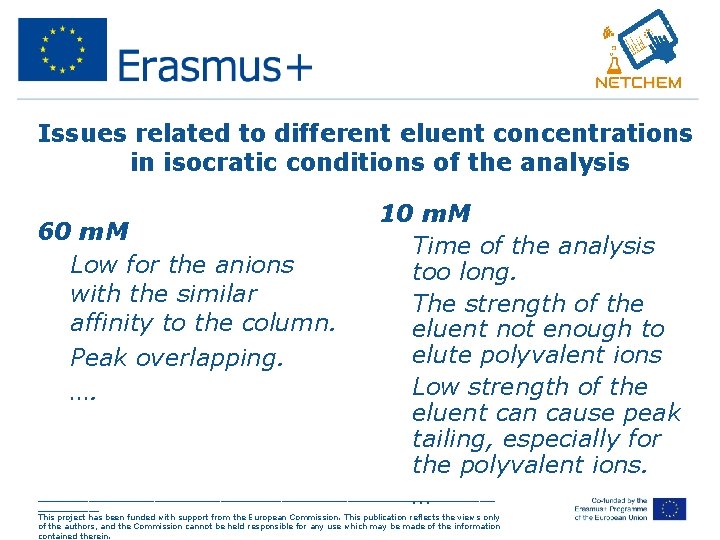
Issues related to different eluent concentrations in isocratic conditions of the analysis 60 m. M • Low for the anions with the similar affinity to the column. • Peak overlapping. • …. 10 m. M • Time of the analysis too long. • The strength of the eluent not enough to elute polyvalent ions • Low strength of the eluent can cause peak tailing, especially for the polyvalent ions. • … _____________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

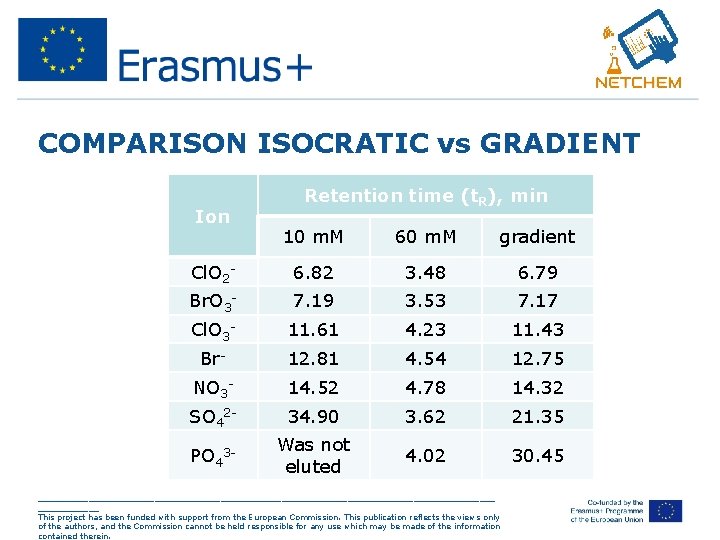
COMPARISON ISOCRATIC vs GRADIENT Ion Retention time (t. R), min 10 m. M 60 m. M gradient Cl. O 2 - 6. 82 3. 48 6. 79 Br. O 3 - 7. 19 3. 53 7. 17 Cl. O 3 - 11. 61 4. 23 11. 43 Br- 12. 81 4. 54 12. 75 NO 3 - 14. 52 4. 78 14. 32 SO 42 - 34. 90 3. 62 21. 35 PO 43 - Was not eluted 4. 02 30. 45 _____________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein.

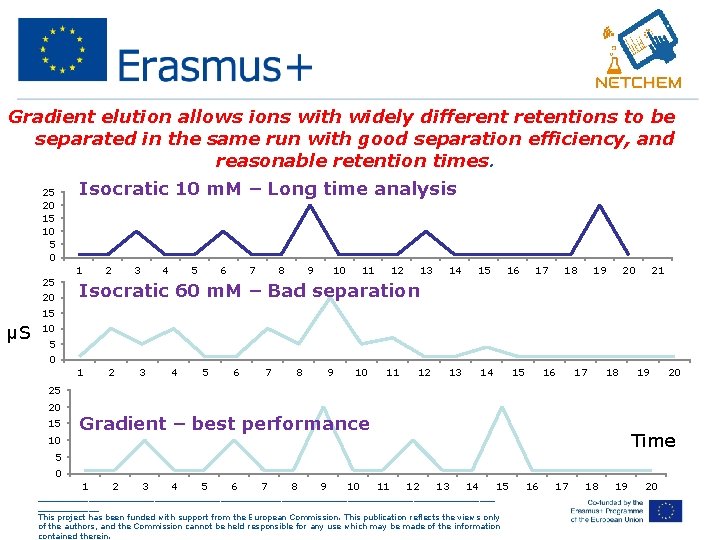
Gradient elution allows ions with widely different retentions to be separated in the same run with good separation efficiency, and reasonable retention times. 25 20 15 10 5 0 25 20 Isocratic 10 m. M – Long time analysis 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 12 13 14 16 17 18 19 20 21 Isocratic 60 m. M – Bad separation 15 μS 10 5 0 1 2 3 4 5 6 7 8 9 10 11 15 16 17 18 19 20 25 20 15 10 Gradient – best performance Time 5 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 _____________________________________________ This project has been funded with support from the European Commission. This publication reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein. 16 17 18 19 20