Part 3 Naming Writing Formulas for Covalent i



- Slides: 9

Part 3: Naming & Writing Formulas for Covalent (i. e. Molecular) Compounds What is a covalent compound? • A compound that is formed when electrons are SHARED between atoms. • This sharing occurs because the electronegativity difference between the atoms is not great enough to result in one atom removing an electron(s) from another atom. • Covalent compounds occur between two non-metals.

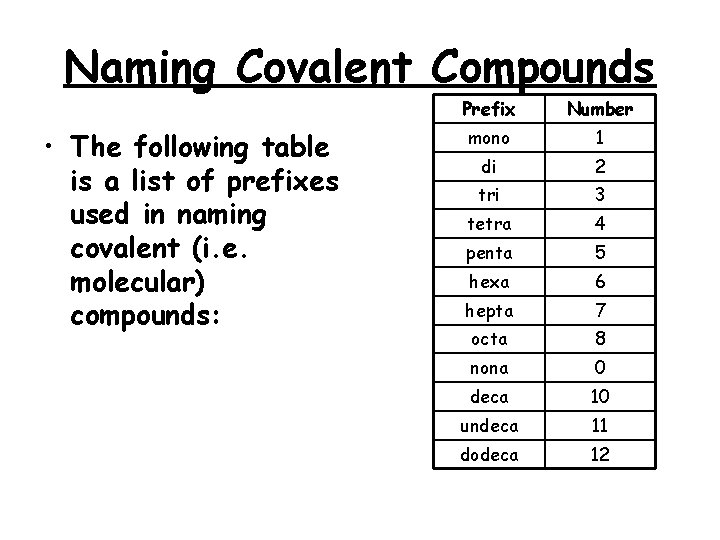
Naming Covalent Compounds • The following table is a list of prefixes used in naming covalent (i. e. molecular) compounds: Prefix Number mono 1 di 2 tri 3 tetra 4 penta 5 hexa 6 hepta 7 octa 8 nona 0 deca 10 undeca 11 dodeca 12

Naming Covalent Compounds (Continued) 1. Name the 1 st element in the formula. 2. Name the 2 nd element in the formula. 3. Remove the ending of the 2 nd element and add “ide”. 4. Add a prefix to each element’s name to indicate how many atoms of each element are present in the compound. - If the 1 st element has only 1 atom, DO NOT add a prefix. - The prefix “mono” is shortened to “mon” if placed before “oxide”.

Examples: 1. Name the following covalent compound: N 2 O 3 steps 1 & 2: step 3: step 4: 2. Name the following covalent compound: CO steps 1 & 2: step 3: step 4:

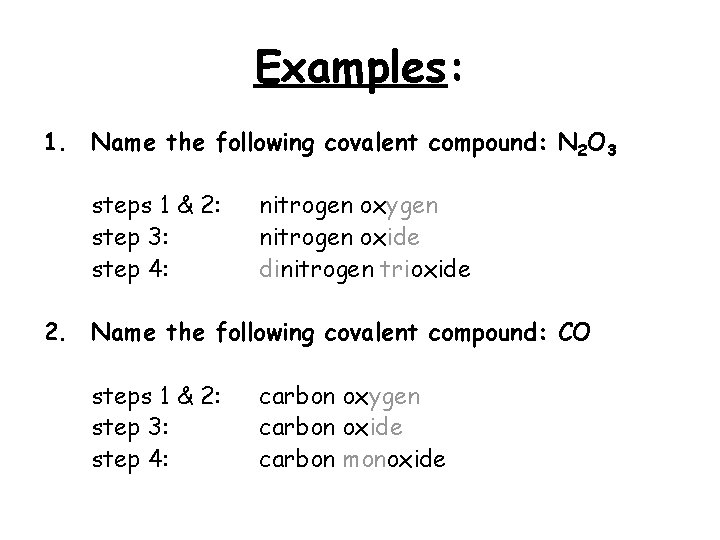
Examples: 1. Name the following covalent compound: N 2 O 3 steps 1 & 2: step 3: step 4: nitrogen oxygen nitrogen oxide dinitrogen trioxide 2. Name the following covalent compound: CO steps 1 & 2: step 3: step 4: carbon oxygen carbon oxide carbon monoxide

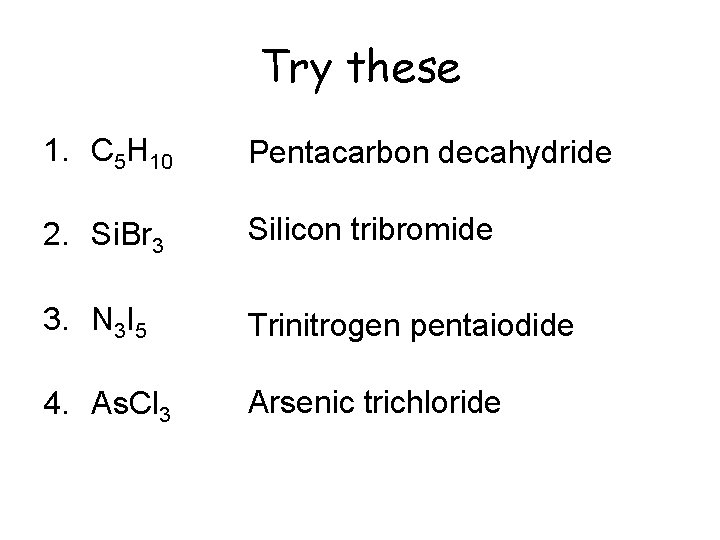
Try these 1. C 5 H 10 Pentacarbon decahydride 2. Si. Br 3 Silicon tribromide 3. N 3 I 5 Trinitrogen pentaiodide 4. As. Cl 3 Arsenic trichloride

Writing Formulas for Covalent Compounds • Refer to the name of the covalent compound to determine the number of atoms present in the chemical formula. • The prefix indicates how many of each atom is present in the compound. • NEVER reduce a formula for a covalent compound. – Example: O 2 F 2 is NOT reduced to OF (as it would be if the compound was ionic).

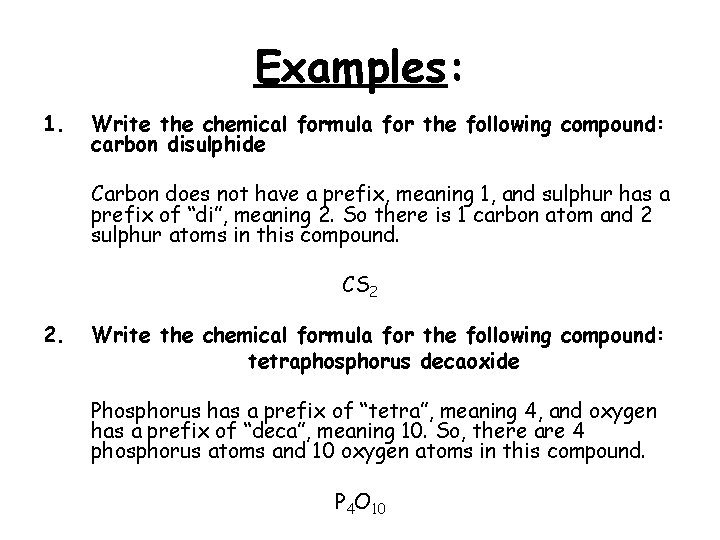
Examples: 1. Write the chemical formula for the following compound: carbon disulphide Carbon does not have a prefix, meaning 1, and sulphur has a prefix of “di”, meaning 2. So there is 1 carbon atom and 2 sulphur atoms in this compound. CS 2 2. Write the chemical formula for the following compound: tetraphosphorus decaoxide Phosphorus has a prefix of “tetra”, meaning 4, and oxygen has a prefix of “deca”, meaning 10. So, there are 4 phosphorus atoms and 10 oxygen atoms in this compound. P 4 O 10

Try these! 1. Pentacarbon tetrahydride C 5 H 4 2. Carbon monoxide CO 3. Sulphur dioxide SO 2 4. Carbon tetrabromide CBr 4