Part 3 iv Substitution Reactions Nucleophile There is
Part 3 iv Substitution Reactions: Nucleophile There is some correlation between basicity and nucleophilicity. remember both a base (B: ) and a nucleophile (Nu: ) are electronically very similar. Both have a lone pair of electrons on an atom within the molecule
Content of Part 3 iv The Nucleophile and SN 1 Reactions The Nucleophile and SN 2 Reactions How to Estimate the Nucleophilicity of a Nucleophile Some Guidelines for Estimating Nucleophilicity Caution on Correlating Basicity with Nucleophilicity! Hard Base/Soft Base

CHM 1 C 3 – Introduction to Chemical Reactivity of Organic Compounds– – Learning Objectives Part 5 iv – Substitution Reactions: Nucleophile After completing PART 4 iv of this course you should have an understanding of, and be able to demonstrate, the following terms, ideas and methods. (i) Changing the Nu: in which the substrate undergoes nucleophilic substitution via an SN 1 mechanism will have no effect on the rate, (ii) Changing the Nu: in which the substrate undergoes nucleophilic substitution via an SN 2 mechanism will have an effect on the rate, (iii) The effectiveness of a nucleophile is dependent on its ability to donate a lone pair of electrons into a s* orbital (could also be a p* orbtial in the case of addition reactions (see a later course)), (iv) p. Ka data can be used as a guide to correlate basicity with nucleophilicity by considering the conjugate base which is equivalent to the nucleophile, (v) Correlations of basicity and nucleophilicity should be done with care as acid/base reactions are thermodynamically controlled and are little effected by steric influences, whereas electrophile/nucleophile reactions are generally under kinetic control and are influenced by steric factors, (vi) Correlations of basicity and nucleophilicity should only be carried out when considering the same heteroatom carrying a lone pair (i. e. compare like with like, Et. O-, Ph. O-, CH 3 C(O)O-). (vii) The concept of hard and soft bases is useful for evaluating the differences in nucleophilicity between different heteroatoms carrying lone pairs, where the assesment is made on the difference in electronegativities of the atoms and the degree of polarisability of the lone pair of electrons, (viii) Some nucleophiles carry lone pairs of electrons on more than one heteroatom and, therefore, can attack electrophilic centres through both heteroatoms – ambident nucleophiles. The more electron rich heteroatom (more lone pairs, higher charge) will react with electrophilic centres involving SN 1 reaction conditions, whilst the less electron rich heteroatom will react with the electrophilic centre under SN 2 conditions, and (ix) One should not forget the role that solvent can have on the nucleophilicity of nucleophiles (see part 4 ii)

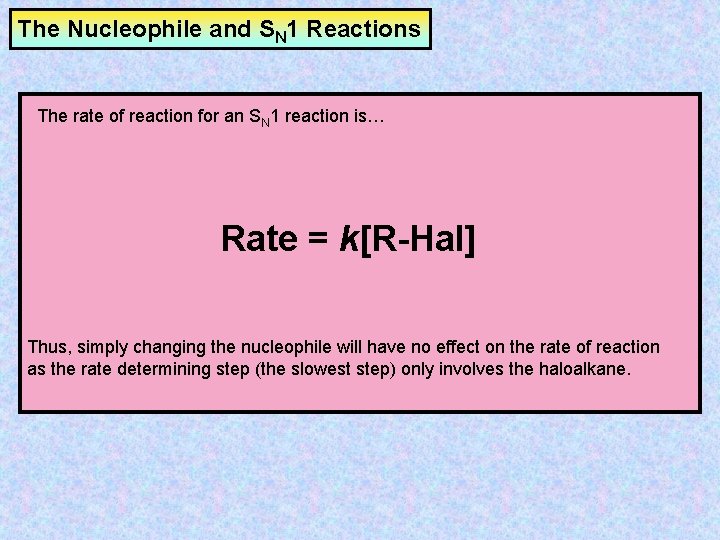
The Nucleophile and SN 1 Reactions The rate of reaction for an SN 1 reaction is… Rate = k[R-Hal] Thus, simply changing the nucleophile will have no effect on the rate of reaction as the rate determining step (the slowest step) only involves the haloalkane.
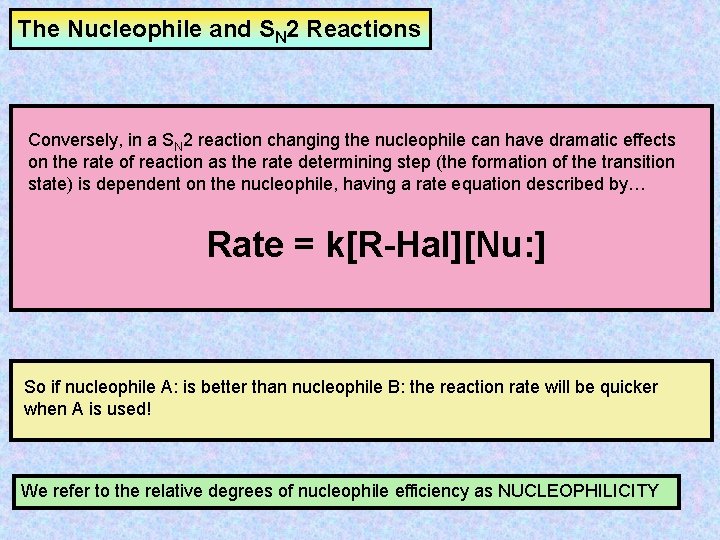
The Nucleophile and SN 2 Reactions Conversely, in a SN 2 reaction changing the nucleophile can have dramatic effects on the rate of reaction as the rate determining step (the formation of the transition state) is dependent on the nucleophile, having a rate equation described by… Rate = k[R-Hal][Nu: ] So if nucleophile A: is better than nucleophile B: the reaction rate will be quicker when A is used! We refer to the relative degrees of nucleophile efficiency as NUCLEOPHILICITY
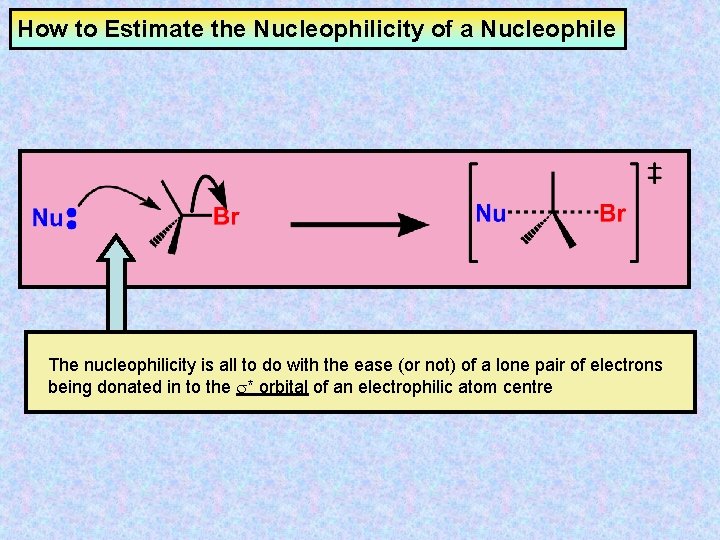
How to Estimate the Nucleophilicity of a Nucleophile The nucleophilicity is all to do with the ease (or not) of a lone pair of electrons being donated in to the s* orbital of an electrophilic atom centre

Some Guidelines for Estimating Nucleophilicity There is some correlation between basicity and nucleophilicity. remember both a base (B: ) and a nucleophile (Nu: ) are electronically very similar. Both have a lone pair of electrons on an atom within the molecule
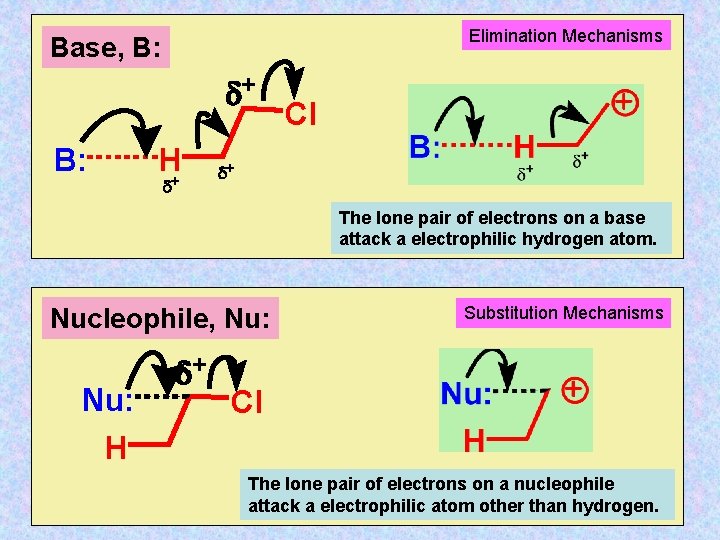
Elimination Mechanisms Base, B: d+ B: H+ d Cl d+ The lone pair of electrons on a base attack a electrophilic hydrogen atom. Nucleophile, Nu: H d+ Substitution Mechanisms Cl The lone pair of electrons on a nucleophile attack a electrophilic atom other than hydrogen.
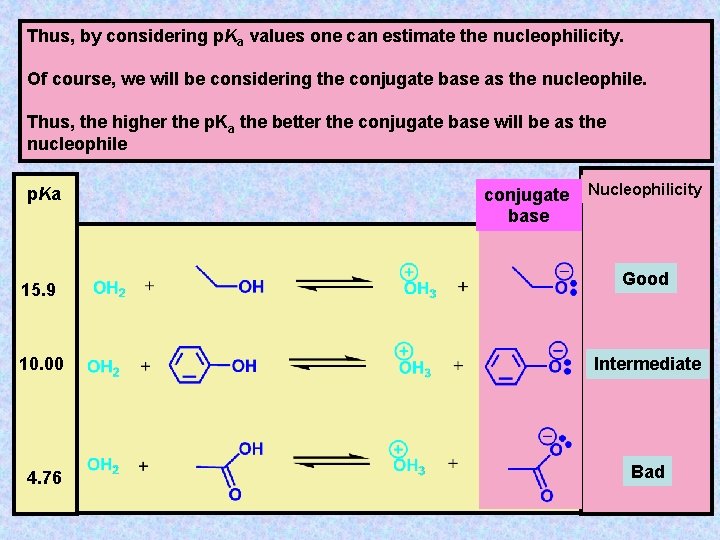
Thus, by considering p. Ka values one can estimate the nucleophilicity. Of course, we will be considering the conjugate base as the nucleophile. Thus, the higher the p. Ka the better the conjugate base will be as the nucleophile p. Ka 15. 9 conjugate base Nucleophilicity Good 10. 00 Intermediate 4. 76 Bad
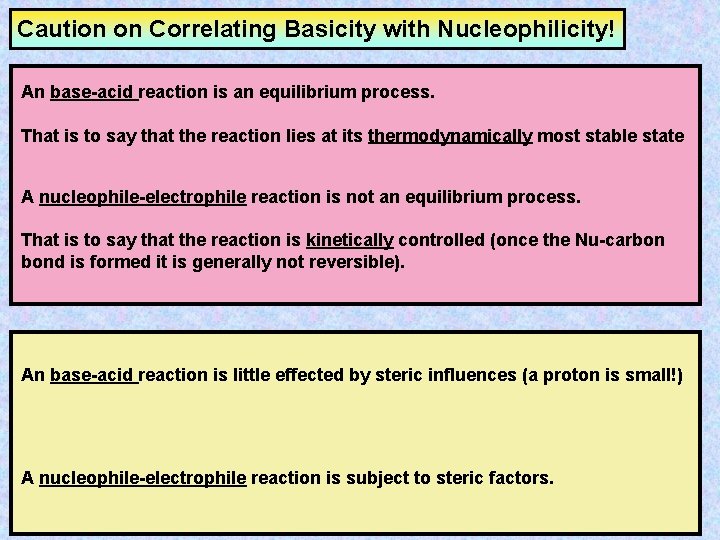
Caution on Correlating Basicity with Nucleophilicity! An base-acid reaction is an equilibrium process. That is to say that the reaction lies at its thermodynamically most stable state A nucleophile-electrophile reaction is not an equilibrium process. That is to say that the reaction is kinetically controlled (once the Nu-carbon bond is formed it is generally not reversible). An base-acid reaction is little effected by steric influences (a proton is small!) A nucleophile-electrophile reaction is subject to steric factors.
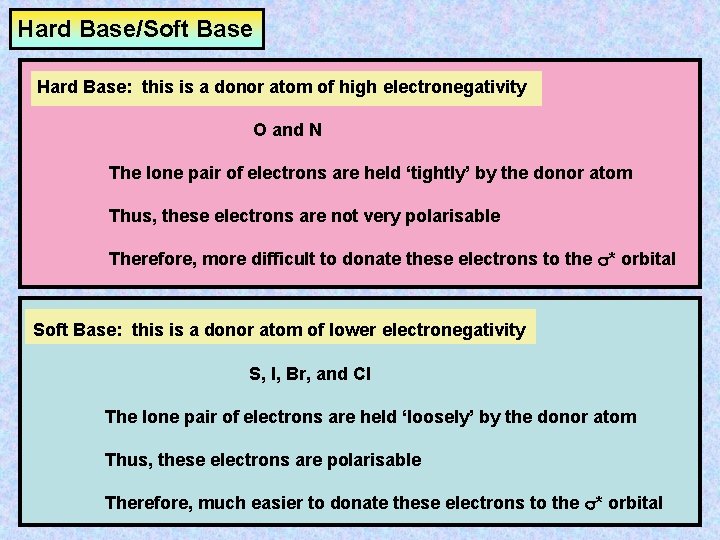
Hard Base/Soft Base Hard Base: this is a donor atom of high electronegativity O and N The lone pair of electrons are held ‘tightly’ by the donor atom Thus, these electrons are not very polarisable Therefore, more difficult to donate these electrons to the s* orbital Soft Base: this is a donor atom of lower electronegativity S, I, Br, and Cl The lone pair of electrons are held ‘loosely’ by the donor atom Thus, these electrons are polarisable Therefore, much easier to donate these electrons to the s* orbital
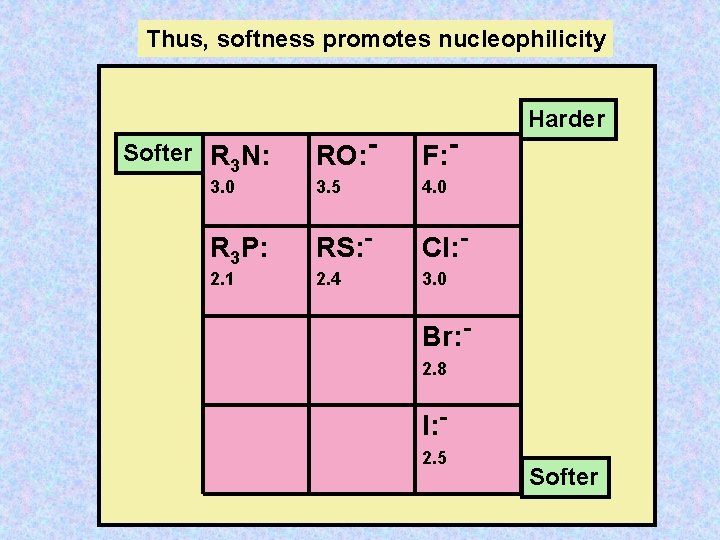
Thus, softness promotes nucleophilicity RO: - F: - 3. 0 3. 5 4. 0 R 3 P: RS: - Cl: - 2. 1 2. 4 3. 0 Softer R 3 N: Harder Br: 2. 8 I: 2. 5 Softer
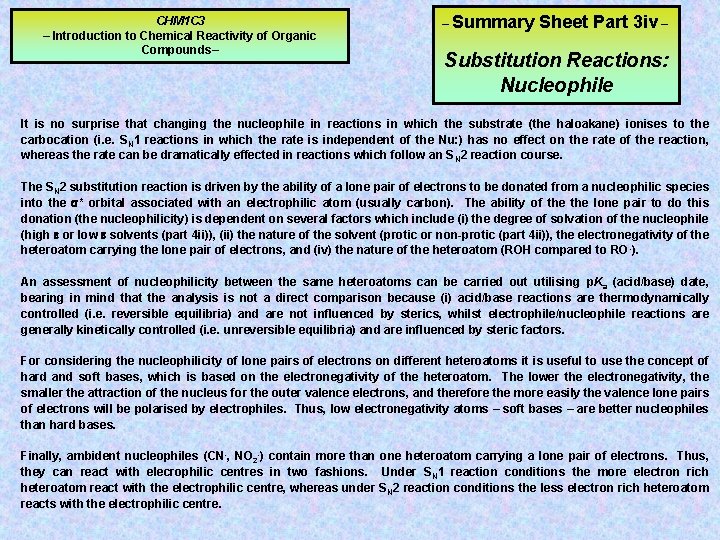
CHM 1 C 3 – Introduction to Chemical Reactivity of Organic Compounds– – Summary Sheet Part 3 iv – Substitution Reactions: Nucleophile It is no surprise that changing the nucleophile in reactions in which the substrate (the haloakane) ionises to the carbocation (i. e. SN 1 reactions in which the rate is independent of the Nu: ) has no effect on the rate of the reaction, whereas the rate can be dramatically effected in reactions which follow an S N 2 reaction course. The SN 2 substitution reaction is driven by the ability of a lone pair of electrons to be donated from a nucleophilic species into the s* orbital associated with an electrophilic atom (usually carbon). The ability of the lone pair to do this donation (the nucleophilicity) is dependent on several factors which include (i) the degree of solvation of the nucleophile (high e or low e solvents (part 4 ii)), (ii) the nature of the solvent (protic or non-protic (part 4 ii)), the electronegativity of the heteroatom carrying the lone pair of electrons, and (iv) the nature of the heteroatom (ROH compared to RO -). An assessment of nucleophilicity between the same heteroatoms can be carried out utilising p. Ka (acid/base) date, bearing in mind that the analysis is not a direct comparison because (i) acid/base reactions are thermodynamically controlled (i. e. reversible equilibria) and are not influenced by sterics, whilst electrophile/nucleophile reactions are generally kinetically controlled (i. e. unreversible equilibria) and are influenced by steric factors. For considering the nucleophilicity of lone pairs of electrons on different heteroatoms it is useful to use the concept of hard and soft bases, which is based on the electronegativity of the heteroatom. The lower the electronegativity, the smaller the attraction of the nucleus for the outer valence electrons, and therefore the more easily the valence lone pairs of electrons will be polarised by electrophiles. Thus, low electronegativity atoms – soft bases – are better nucleophiles than hard bases. Finally, ambident nucleophiles (CN-, NO 2 -) contain more than one heteroatom carrying a lone pair of electrons. Thus, they can react with elecrophilic centres in two fashions. Under SN 1 reaction conditions the more electron rich heteroatom react with the electrophilic centre, whereas under SN 2 reaction conditions the less electron rich heteroatom reacts with the electrophilic centre.
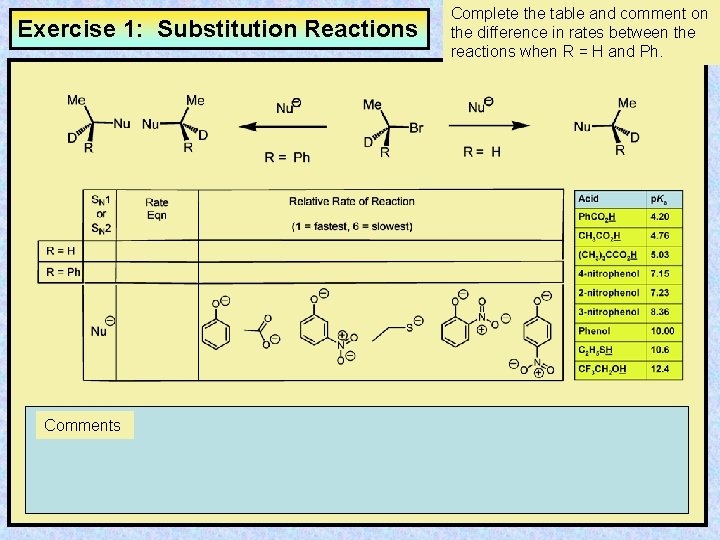
Exercise 1: Substitution Reactions Comments Complete the table and comment on the difference in rates between the reactions when R = H and Ph.
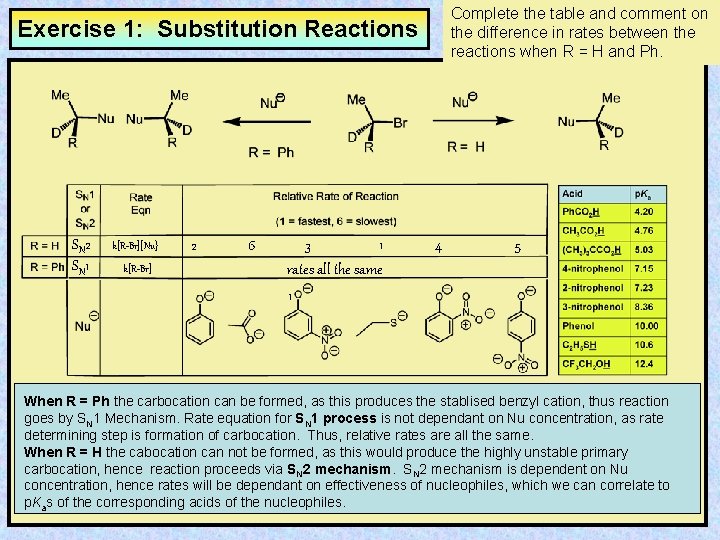
Complete the table and comment on the difference in rates between the reactions when R = H and Ph. Exercise 1: Substitution Reactions SN 2 SN 1 k[R-Br][Nu} k[R-Br] 2 6 1 3 rates all the same 1 4 5 When R = Ph the carbocation can be formed, as this produces the stablised benzyl cation, thus reaction goes by SN 1 Mechanism. Rate equation for SN 1 process is not dependant on Nu concentration, as rate determining step is formation of carbocation. Thus, relative rates are all the same. When R = H the cabocation can not be formed, as this would produce the highly unstable primary carbocation, hence reaction proceeds via SN 2 mechanism is dependent on Nu concentration, hence rates will be dependant on effectiveness of nucleophiles, which we can correlate to p. Kas of the corresponding acids of the nucleophiles.
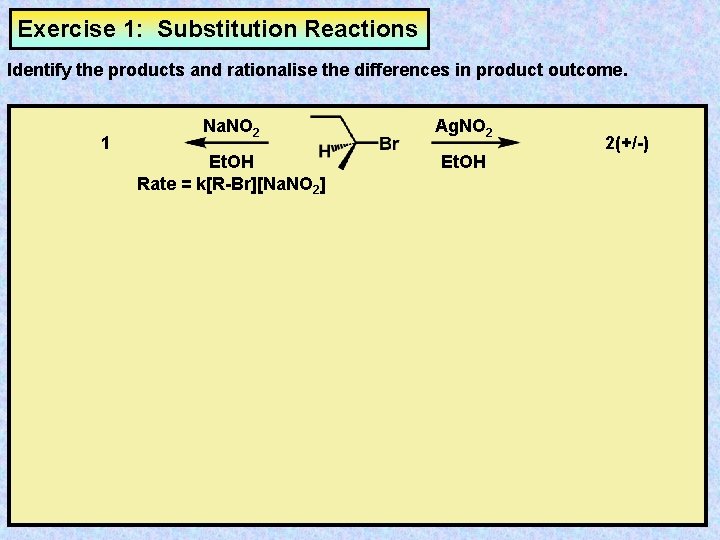
Exercise 1: Substitution Reactions Identify the products and rationalise the differences in product outcome. 1 Na. NO 2 Ag. NO 2 Et. OH Rate = k[R-Br][Na. NO 2] Et. OH 2(+/-)
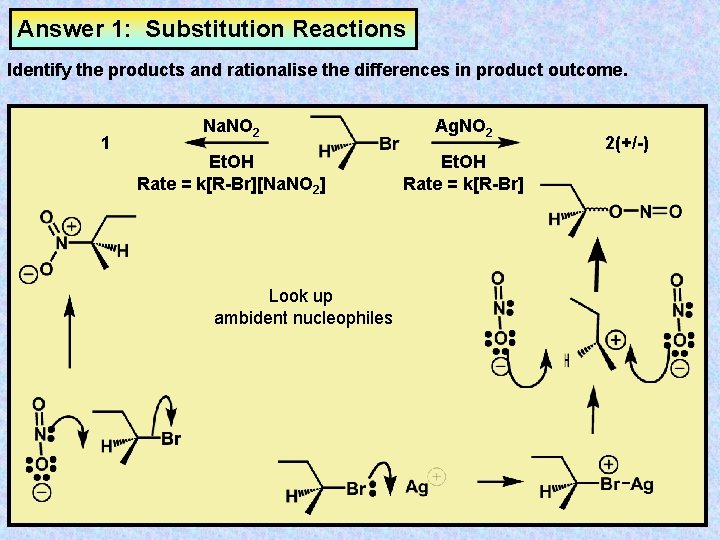
Answer 1: Substitution Reactions Identify the products and rationalise the differences in product outcome. 1 Na. NO 2 Ag. NO 2 Et. OH Rate = k[R-Br][Na. NO 2] Et. OH Rate = k[R-Br] Look up ambident nucleophiles 2(+/-)
- Slides: 19