Part 1 Naming inorganic Compounds and Writing Formulas





- Slides: 46

Part 1 Naming inorganic Compounds and Writing Formulas

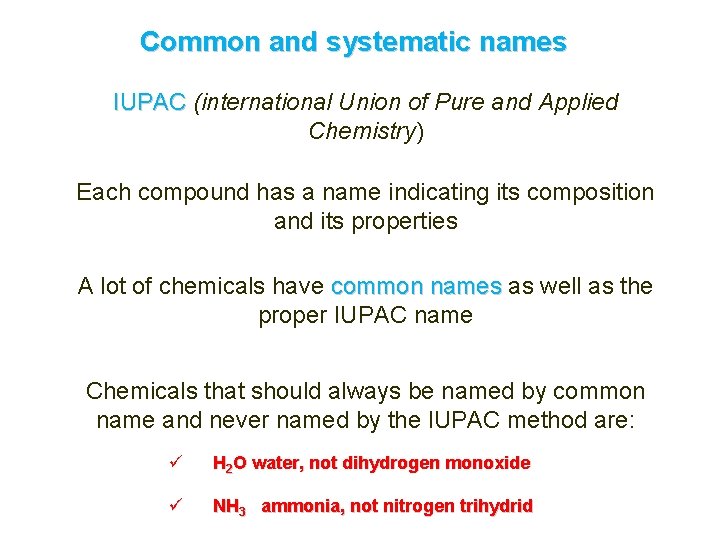
Common and systematic names IUPAC (international Union of Pure and Applied Chemistry) Each compound has a name indicating its composition and its properties A lot of chemicals have common names as well as the proper IUPAC name Chemicals that should always be named by common name and never named by the IUPAC method are: ü H 2 O water, not dihydrogen monoxide ü NH 3 ammonia, not nitrogen trihydrid

To keep in mind!

1. Rules to assign Oxidation Numbers (O. N. ) 2. Classification of molecules 3. Rules to assign names and write formula 4. Chemical reactions balancing equations

1. Oxidation number of an element represents the positive or negative character of an atom of that element in a particular bonding situation

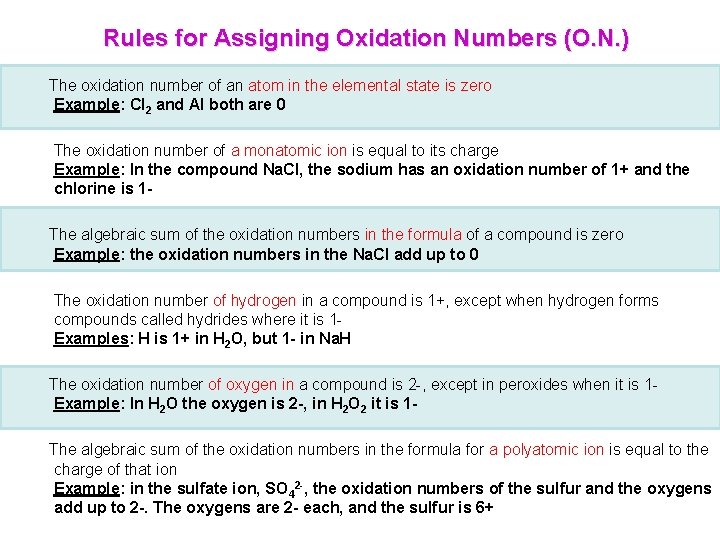
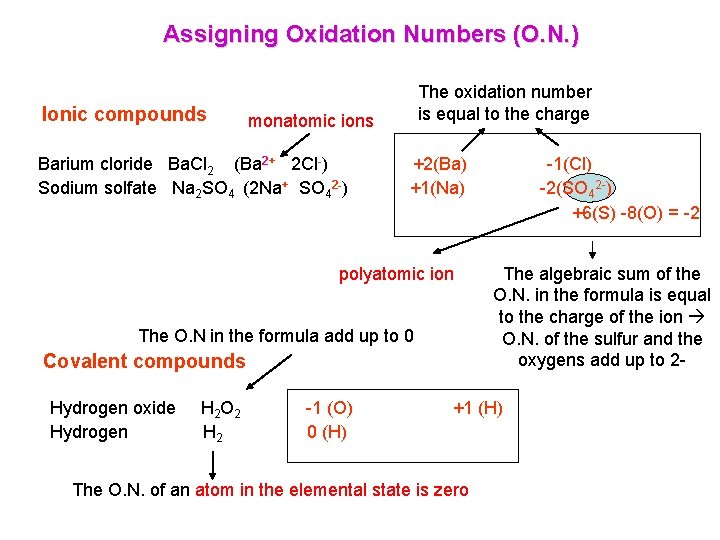
Rules for Assigning Oxidation Numbers (O. N. ) The oxidation number of an atom in the elemental state is zero Example: Cl 2 and Al both are 0 The oxidation number of a monatomic ion is equal to its charge Example: In the compound Na. Cl, the sodium has an oxidation number of 1+ and the chlorine is 1 The algebraic sum of the oxidation numbers in the formula of a compound is zero Example: the oxidation numbers in the Na. Cl add up to 0 The oxidation number of hydrogen in a compound is 1+, except when hydrogen forms compounds called hydrides where it is 1 Examples: H is 1+ in H 2 O, but 1 - in Na. H The oxidation number of oxygen in a compound is 2 -, except in peroxides when it is 1 Example: In H 2 O the oxygen is 2 -, in H 2 O 2 it is 1 The algebraic sum of the oxidation numbers in the formula for a polyatomic ion is equal to the charge of that ion Example: in the sulfate ion, SO 42 -, the oxidation numbers of the sulfur and the oxygens add up to 2 -. The oxygens are 2 - each, and the sulfur is 6+

Assigning Oxidation Numbers (O. N. ) Ionic compounds The oxidation number is equal to the charge monatomic ions Barium cloride Ba. Cl 2 (Ba 2+ 2 Cl-) Sodium solfate Na 2 SO 4 (2 Na+ SO 42 -) +2(Ba) +1(Na) polyatomic ion The O. N in the formula add up to 0 Covalent compounds Hydrogen oxide Hydrogen H 2 O 2 H 2 -1 (O) 0 (H) -1(Cl) -2(SO 42 -) +6(S) -8(O) = -2 The algebraic sum of the O. N. in the formula is equal to the charge of the ion O. N. of the sulfur and the oxygens add up to 2 - +1 (H) The O. N. of an atom in the elemental state is zero

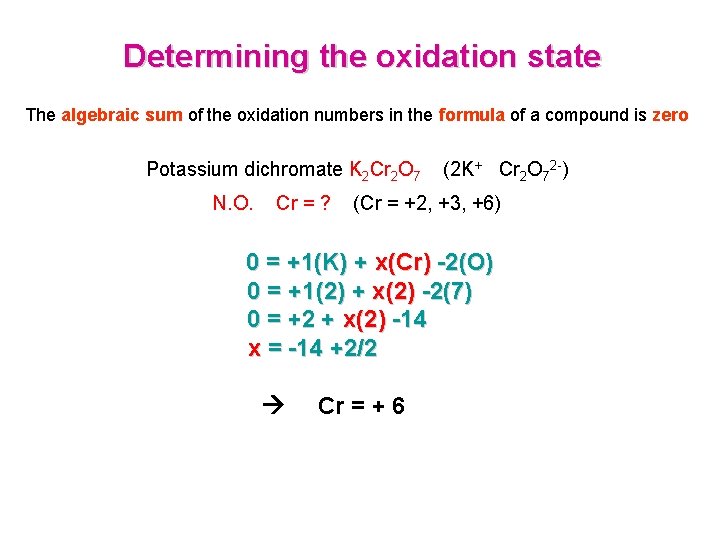
Determining the oxidation state The algebraic sum of the oxidation numbers in the formula of a compound is zero Potassium dichromate K 2 Cr 2 O 7 N. O. Cr = ? (2 K+ Cr 2 O 72 -) (Cr = +2, +3, +6) 0 = +1(K) + x(Cr) -2(O) 0 = +1(2) + x(2) -2(7) 0 = +2 + x(2) -14 x = -14 +2/2 Cr = + 6

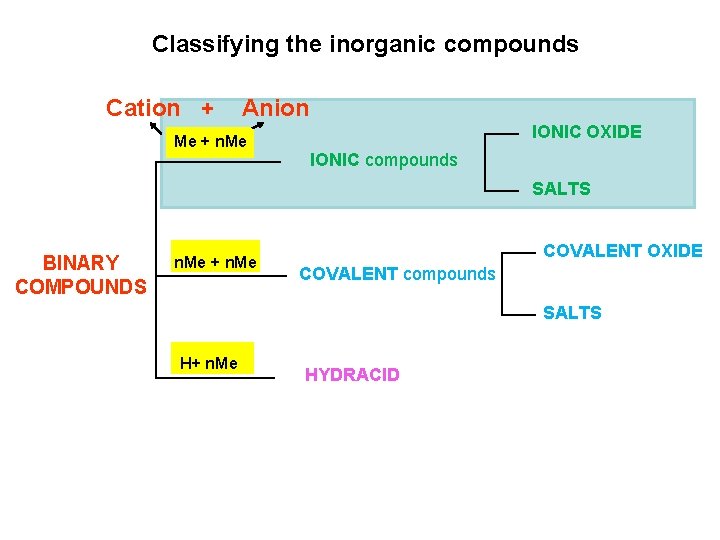
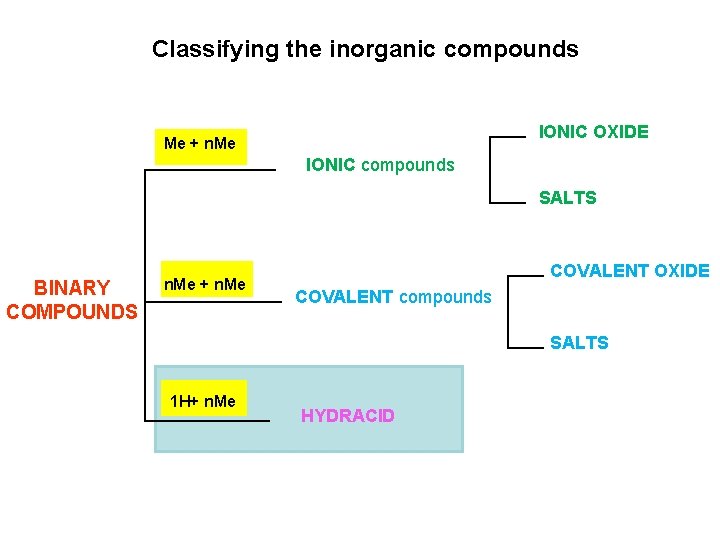
Classifying the inorganic compounds ØBINARY COMPOUNDS composed of just two elements ØTERNARY COMPOUNDS containing three or more elements

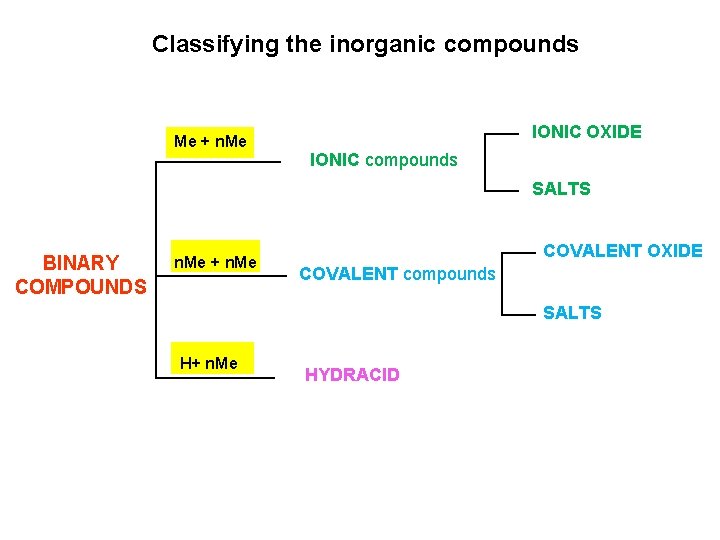
Classifying the inorganic compounds Me + n. Me IONIC OXIDE IONIC compounds SALTS BINARY COMPOUNDS n. Me + n. Me COVALENT OXIDE COVALENT compounds SALTS H+ n. Me HYDRACID

Classifying the inorganic compounds Cation + Anion Me + n. Me IONIC OXIDE IONIC compounds SALTS BINARY COMPOUNDS n. Me + n. Me COVALENT OXIDE COVALENT compounds SALTS H+ n. Me HYDRACID

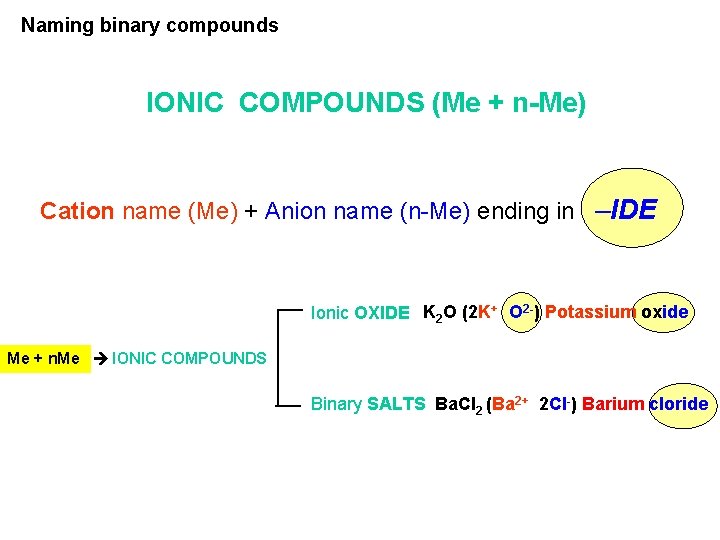
Naming binary compounds IONIC COMPOUNDS (Me + n-Me) Cation name (Me) + Anion name (n-Me) ending in –IDE Ionic OXIDE K 2 O (2 K+ O 2 -) Potassium oxide Me + n. Me IONIC COMPOUNDS Binary SALTS Ba. Cl 2 (Ba 2+ 2 Cl-) Barium cloride

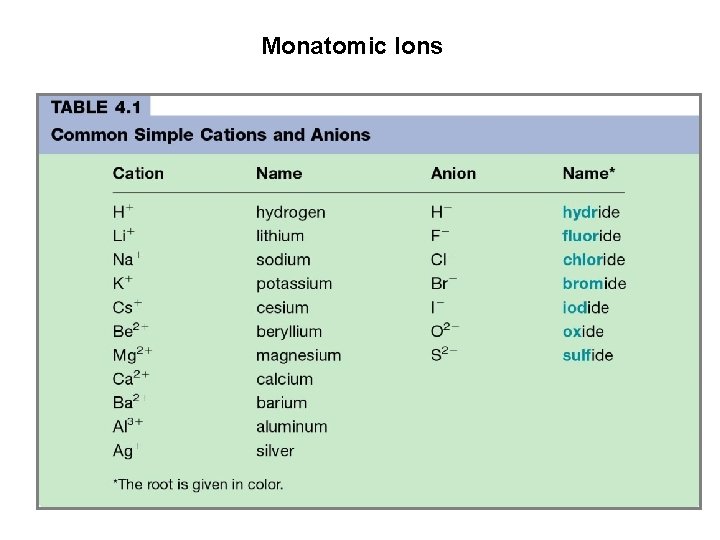
Monatomic Ions

Naming binary compounds IONIC COMPOUNDS When the METALS can assume more than one oxidation state……

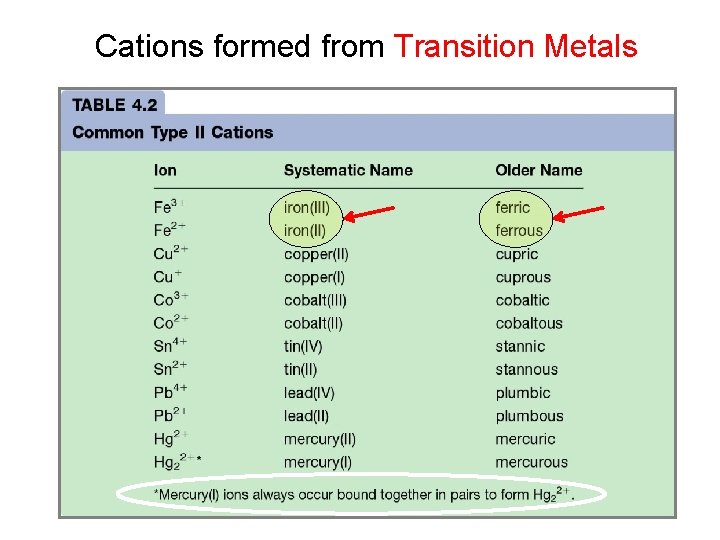
Cations formed from Transition Metals

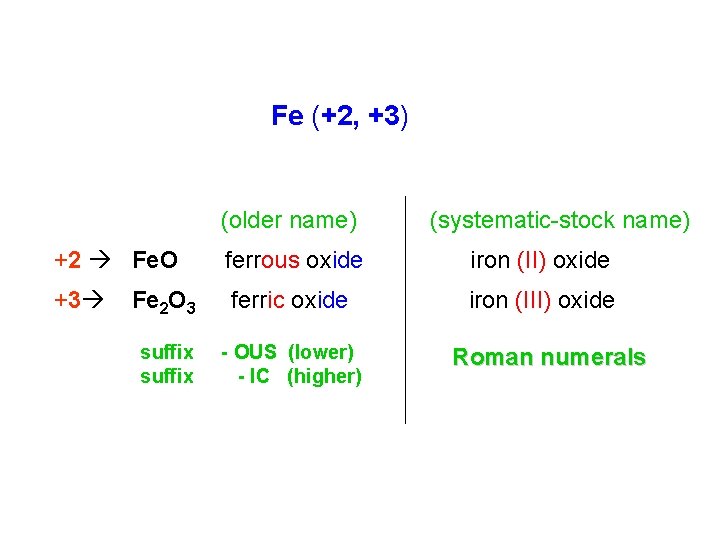
Fe (+2, +3) (older name) (systematic-stock name) +2 Fe. O ferrous oxide iron (II) oxide +3 Fe 2 O 3 ferric oxide iron (III) oxide suffix - OUS (lower) - IC (higher) Roman numerals

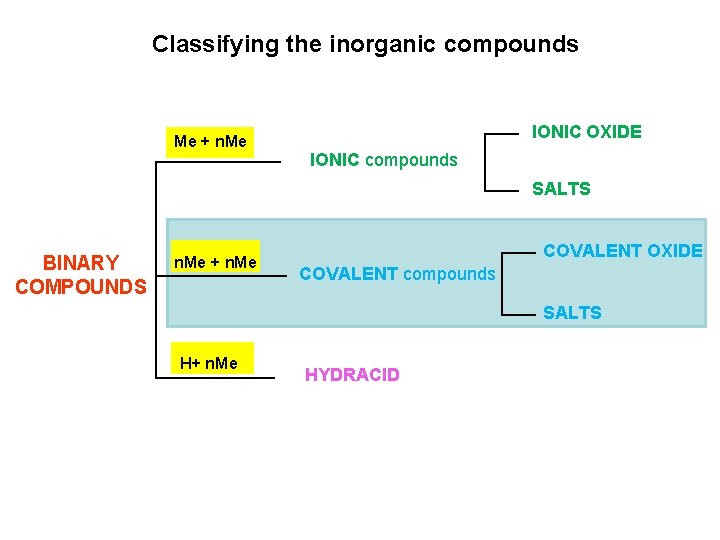
Classifying the inorganic compounds IONIC OXIDE Me + n. Me IONIC compounds SALTS BINARY COMPOUNDS n. Me + n. Me COVALENT OXIDE COVALENT compounds SALTS 1 H+ n. Me HYDRACID

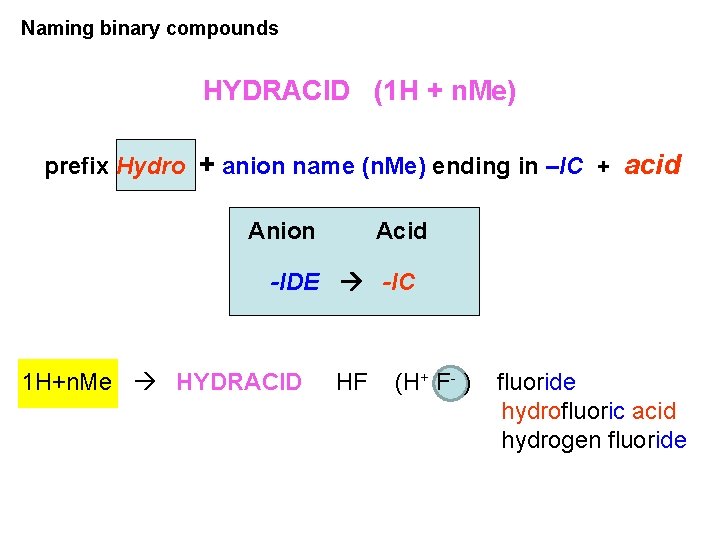
Naming binary compounds HYDRACID (1 H + n. Me) prefix Hydro + anion name (n. Me) ending in –IC + acid Anion Acid -IDE -IC 1 H+n. Me HYDRACID HF (H+ F- ) fluoride hydrofluoric acid hydrogen fluoride

Classifying the inorganic compounds Me + n. Me IONIC OXIDE IONIC compounds SALTS BINARY COMPOUNDS n. Me + n. Me COVALENT OXIDE COVALENT compounds SALTS H+ n. Me HYDRACID

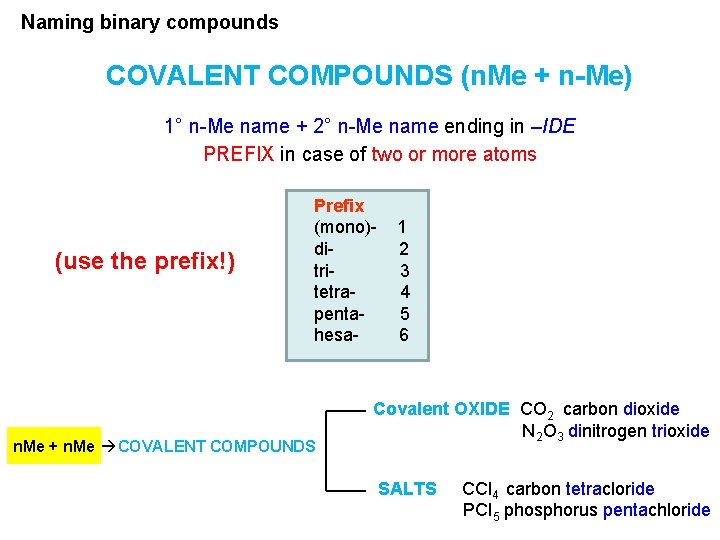
Naming binary compounds COVALENT COMPOUNDS (n. Me + n-Me) 1° n-Me name + 2° n-Me name ending in –IDE PREFIX in case of two or more atoms (use the prefix!) Prefix (mono)ditritetrapentahesa- n. Me + n. Me COVALENT COMPOUNDS 1 2 3 4 5 6 Covalent OXIDE CO 2 carbon dioxide N 2 O 3 dinitrogen trioxide SALTS CCl 4 carbon tetracloride PCl 5 phosphorus pentachloride

Si B P H C S I Br N Cl O The elemet that occurs first in this series is written and name first, the neme of the second element retains the -IDE ending F

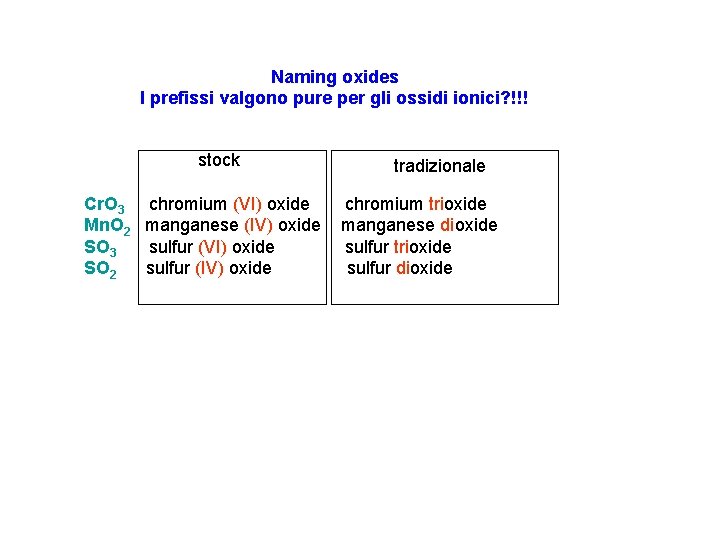
Naming oxides I prefissi valgono pure per gli ossidi ionici? !!! stock Cr. O 3 chromium (VI) oxide Mn. O 2 manganese (IV) oxide SO 3 sulfur (VI) oxide SO 2 sulfur (IV) oxide tradizionale chromium trioxide manganese dioxide sulfur trioxide sulfur dioxide

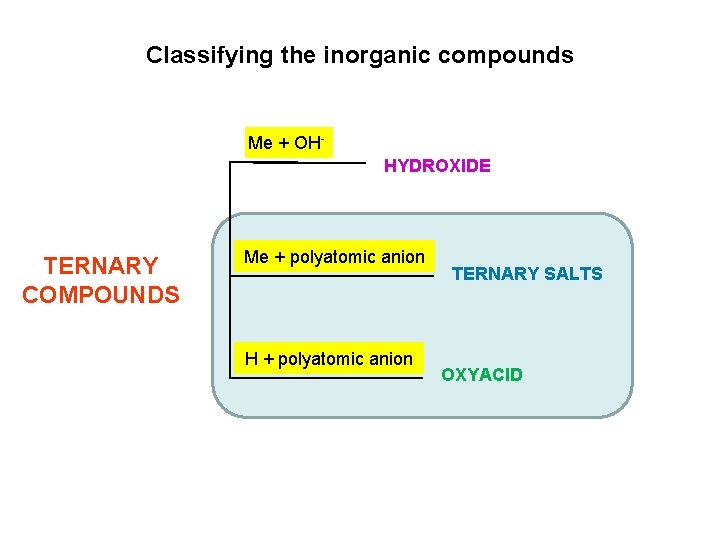
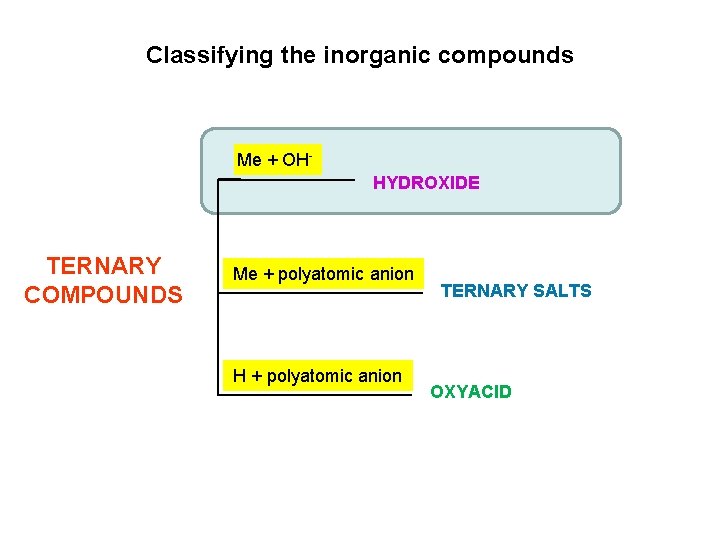
Classifying the inorganic compounds Me + OHHYDROXIDE TERNARY COMPOUNDS Me + polyatomic anion H + polyatomic anion TERNARY SALTS OXYACID

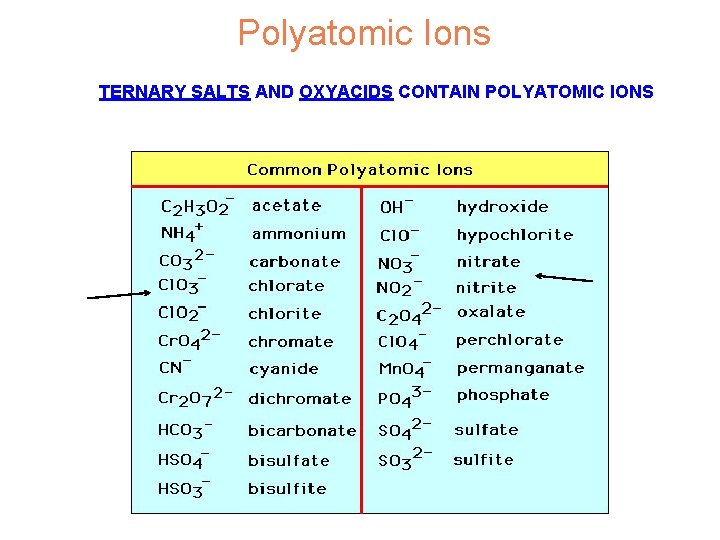
Polyatomic Ions TERNARY SALTS AND OXYACIDS CONTAIN POLYATOMIC IONS

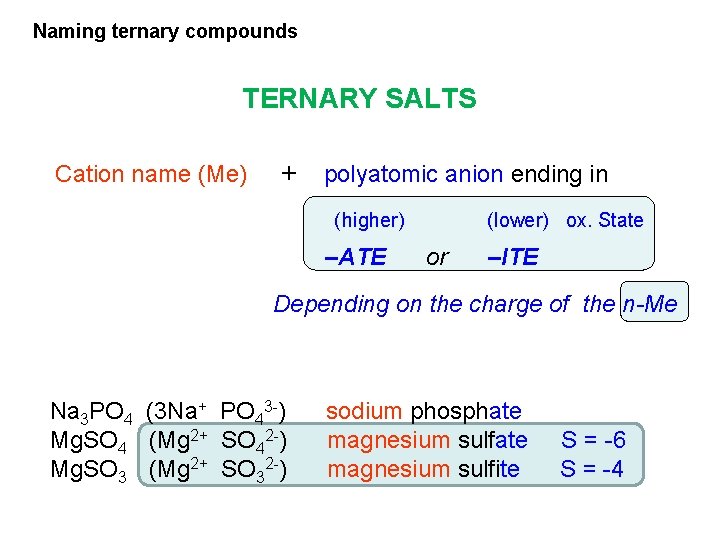
Naming ternary compounds TERNARY SALTS Cation name (Me) + polyatomic anion ending in (higher) –ATE (lower) ox. State or –ITE Depending on the charge of the n-Me Na 3 PO 4 (3 Na+ PO 43 -) Mg. SO 4 (Mg 2+ SO 42 -) Mg. SO 3 (Mg 2+ SO 32 -) sodium phosphate magnesium sulfite S = -6 S = -4

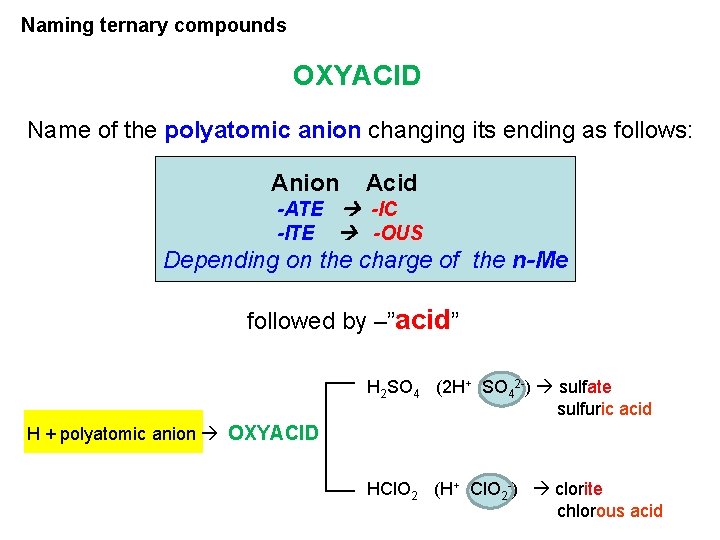
Naming ternary compounds OXYACID Name of the polyatomic anion changing its ending as follows: Anion Acid -ATE -IC -ITE -OUS Depending on the charge of the n-Me followed by –”acid” H 2 SO 4 (2 H+ SO 42 -) sulfate sulfuric acid H + polyatomic anion OXYACID HCl. O 2 (H+ Cl. O 2 -) clorite chlorous acid

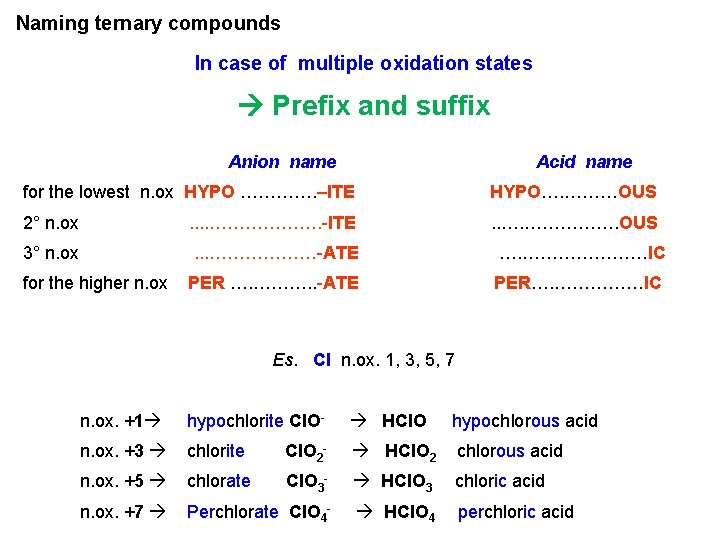
Naming ternary compounds In case of multiple oxidation states Prefix and suffix Anion name Acid name for the lowest n. ox HYPO …………. –ITE HYPO…. ………OUS 2° n. ox . . ………………. -ITE . . …. ……………. OUS 3° n. ox . . . ………………-ATE …. …………………IC PER …. ………. . -ATE PER…. ……………IC for the higher n. ox Es. Cl n. ox. 1, 3, 5, 7 n. ox. +1 hypochlorite Cl. O- HCIO hypochlorous acid n. ox. +3 chlorite Cl. O 2 - HCl. O 2 chlorous acid n. ox. +5 chlorate Cl. O 3 - HCl. O 3 chloric acid n. ox. +7 Perchlorate Cl. O 4 - HCl. O 4 perchloric acid

Classifying the inorganic compounds Me + OHHYDROXIDE TERNARY COMPOUNDS Me + polyatomic anion H + polyatomic anion TERNARY SALTS OXYACID

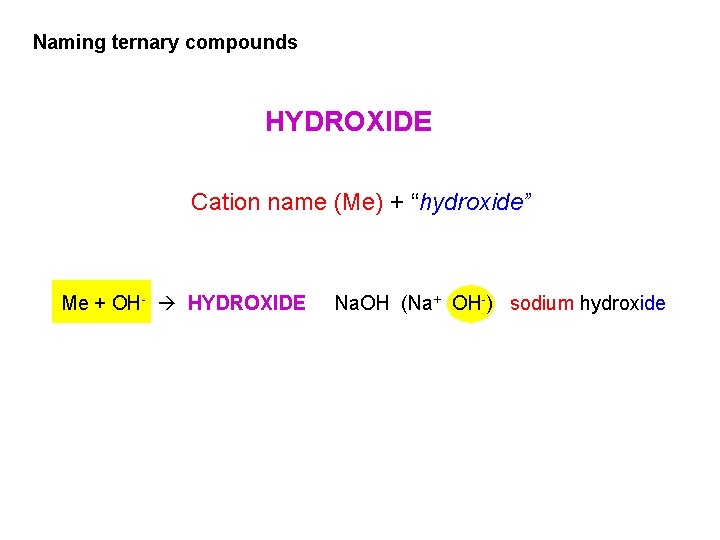
Naming ternary compounds HYDROXIDE Cation name (Me) + “hydroxide” Me + OH- HYDROXIDE Na. OH (Na+ OH-) sodium hydroxide

Part 2 Chemical reaction: Features and Balancing

Chemical equation One substance changes chemically into another substance Reactants starting materials Products resulting substance Additional information in a chemical reaction: §Physical state of the substance (solid, liquid, or gas) §Identifies the solvent, if there is one (a solvent is the solution the materials are dissolved in, such as water) §Experimental conditions such as heat, light, or electrical energy added

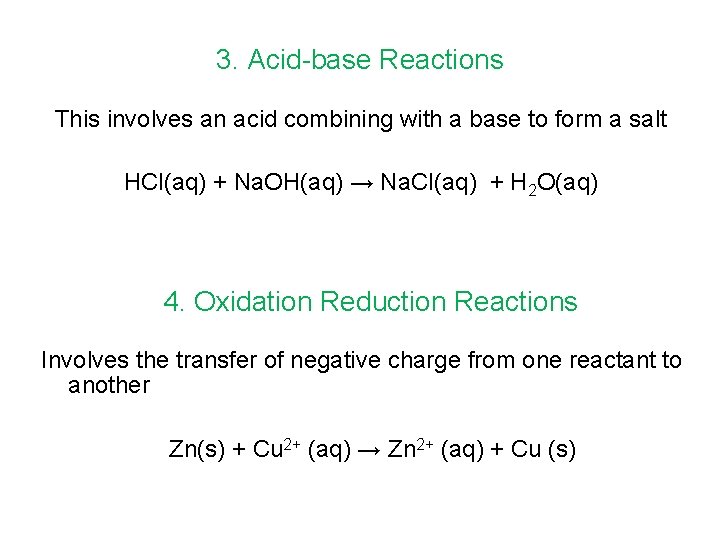
Types of Chemical Reactions There are four main types of chemical reactions 1. Precipitation reactions 2. Reactions with Oxygen 3. Acid-base reactions 4. Oxidation-reduction reactions

1. Precipitation reactions • A chemical change that produces an insoluble product that will form a solid. • Usually the solid can be seen “falling out” of the solution, hence, called precipitation. At other times the solid makes the solution turn from clear to cloudy. 2. Reactions with oxygen • Many substances react with oxygen. • If the substance contains carbon then carbon dioxide is usually produced. • If the substance contains hydrogen, then water is usually produced.

3. Acid-base Reactions This involves an acid combining with a base to form a salt HCl(aq) + Na. OH(aq) → Na. Cl(aq) + H 2 O(aq) 4. Oxidation Reduction Reactions Involves the transfer of negative charge from one reactant to another Zn(s) + Cu 2+ (aq) → Zn 2+ (aq) + Cu (s)

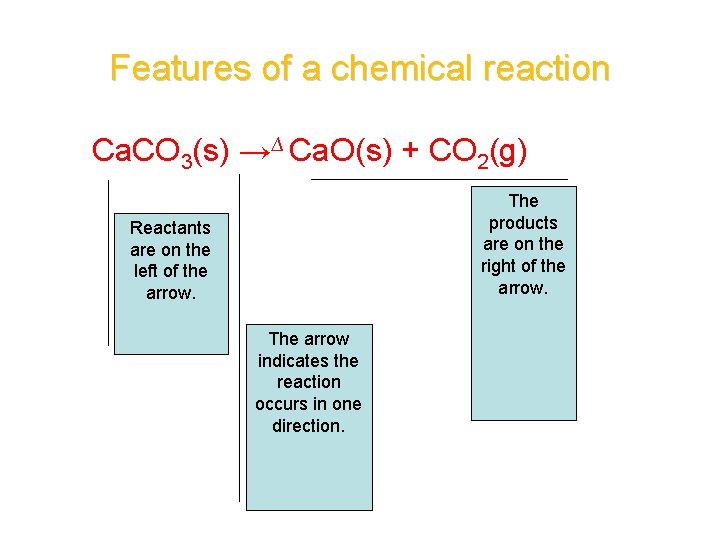
Features of a chemical reaction Ca. CO 3(s) →∆ Ca. O(s) + CO 2(g) The products are on the right of the arrow. Reactants are on the left of the arrow. The arrow indicates the reaction occurs in one direction.

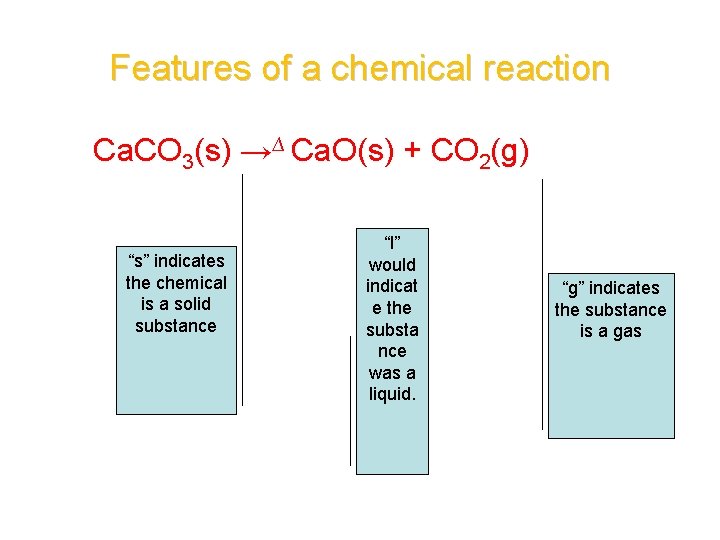
Features of a chemical reaction Ca. CO 3(s) →∆ Ca. O(s) + CO 2(g) “s” indicates the chemical is a solid substance “l” would indicat e the substa nce was a liquid. “g” indicates the substance is a gas

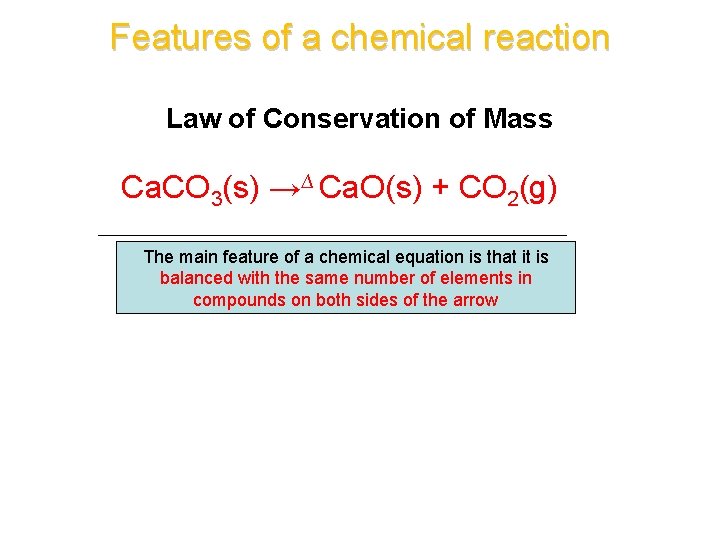
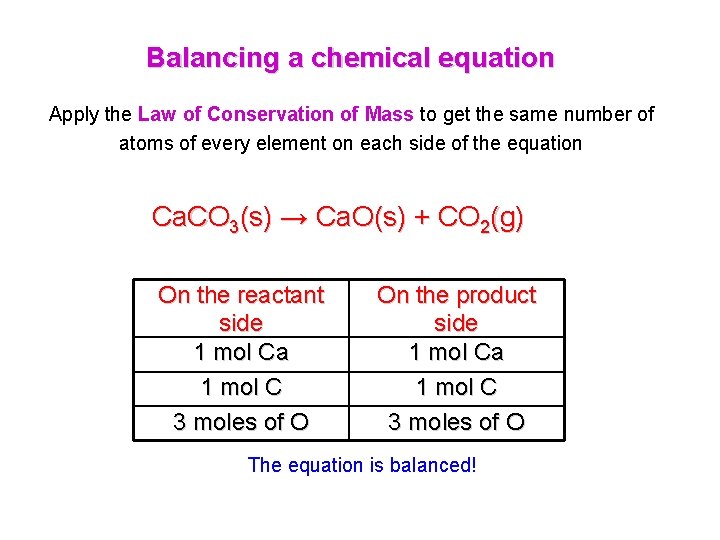
Features of a chemical reaction Law of Conservation of Mass Ca. CO 3(s) →∆ Ca. O(s) + CO 2(g) The main feature of a chemical equation is that it is balanced with the same number of elements in compounds on both sides of the arrow

Balancing a chemical equation Apply the Law of Conservation of Mass to get the same number of atoms of every element on each side of the equation Ca. CO 3(s) → Ca. O(s) + CO 2(g) On the reactant side 1 mol Ca 1 mol C 3 moles of O On the product side 1 mol Ca 1 mol C 3 moles of O The equation is balanced!

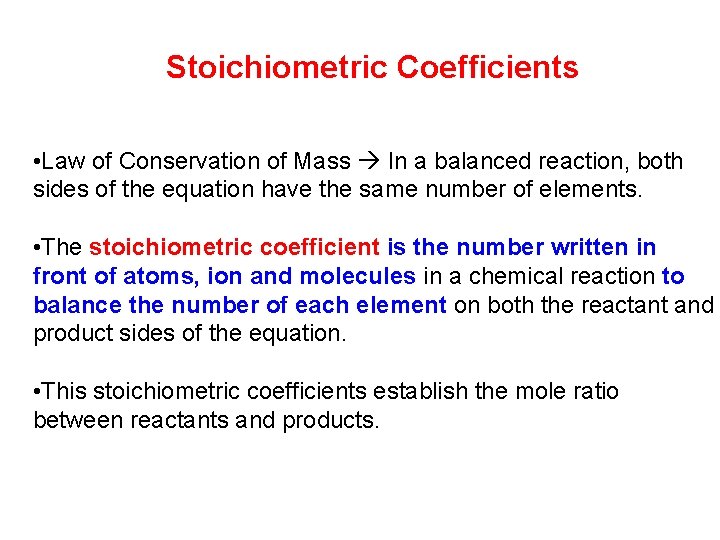
Stoichiometric Coefficients • Law of Conservation of Mass In a balanced reaction, both sides of the equation have the same number of elements. • The stoichiometric coefficient is the number written in front of atoms, ion and molecules in a chemical reaction to balance the number of each element on both the reactant and product sides of the equation. • This stoichiometric coefficients establish the mole ratio between reactants and products.

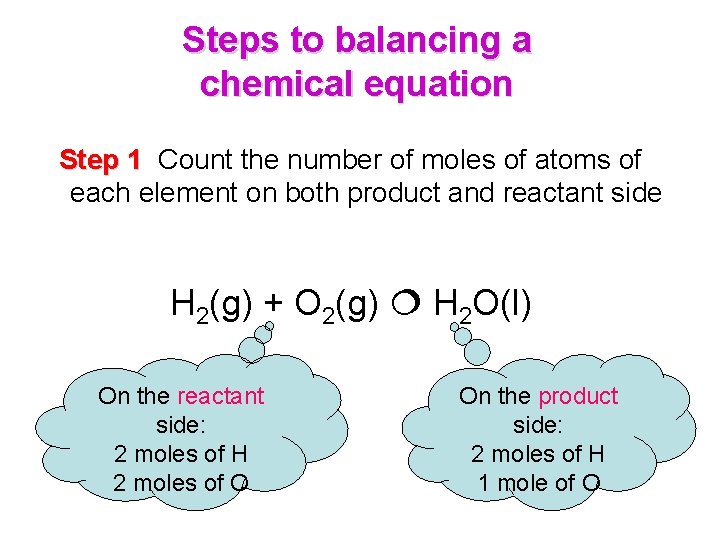
Steps to balancing a chemical equation Step 1 Count the number of moles of atoms of each element on both product and reactant side H 2(g) + O 2(g) H 2 O(l) On the reactant side: 2 moles of H 2 moles of O On the product side: 2 moles of H 1 mole of O

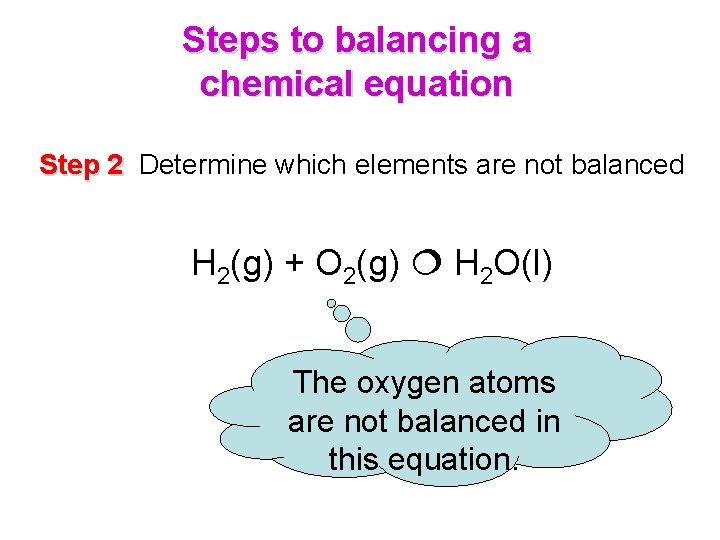
Steps to balancing a chemical equation Step 2 Determine which elements are not balanced H 2(g) + O 2(g) H 2 O(l) The oxygen atoms are not balanced in this equation.

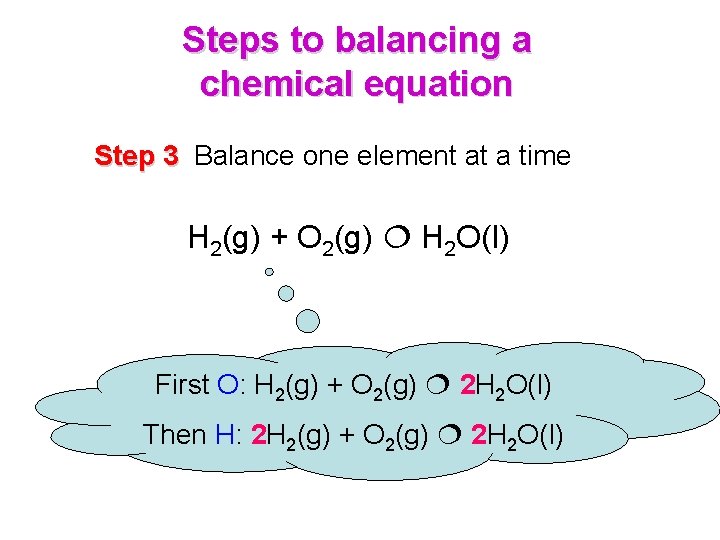
Steps to balancing a chemical equation Step 3 Balance one element at a time H 2(g) + O 2(g) H 2 O(l) First O: H 2(g) + O 2(g) 2 H 2 O(l) Then H: 2 H 2(g) + O 2(g) 2 H 2 O(l)

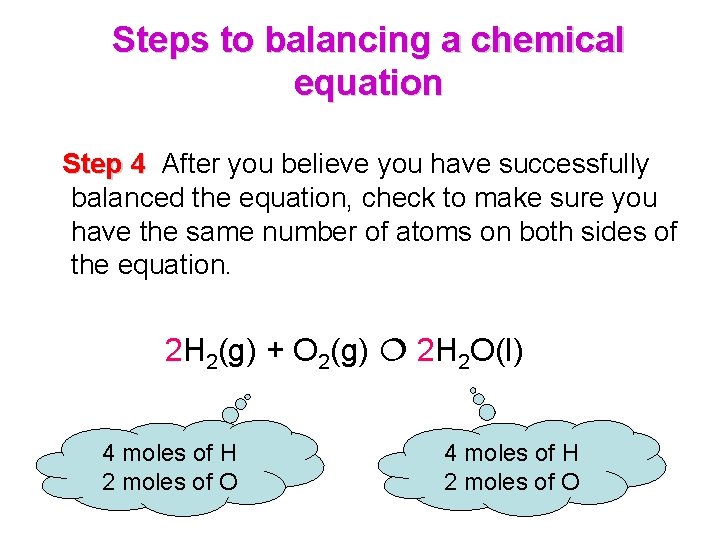
Steps to balancing a chemical equation Step 4 After you believe you have successfully balanced the equation, check to make sure you have the same number of atoms on both sides of the equation. 2 H 2(g) + O 2(g) 2 H 2 O(l) 4 moles of H 2 moles of O

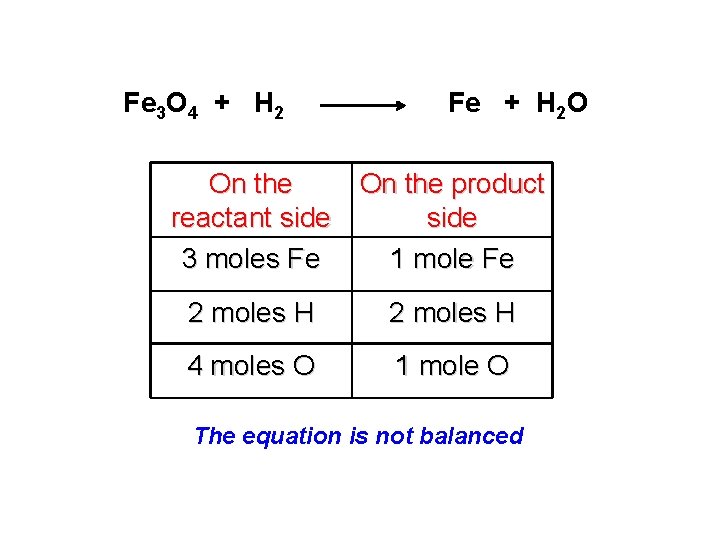
Fe 3 O 4 + H 2 Fe + H 2 O On the product reactant side 3 moles Fe 1 mole Fe 2 moles H 4 moles O 1 mole O The equation is not balanced

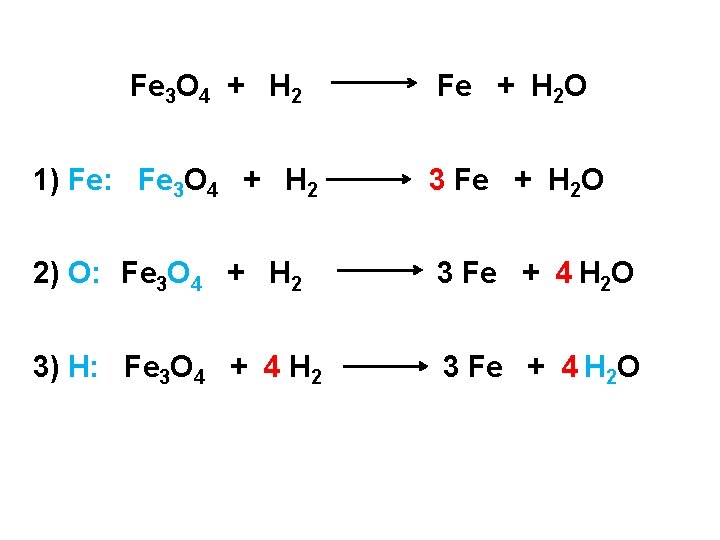
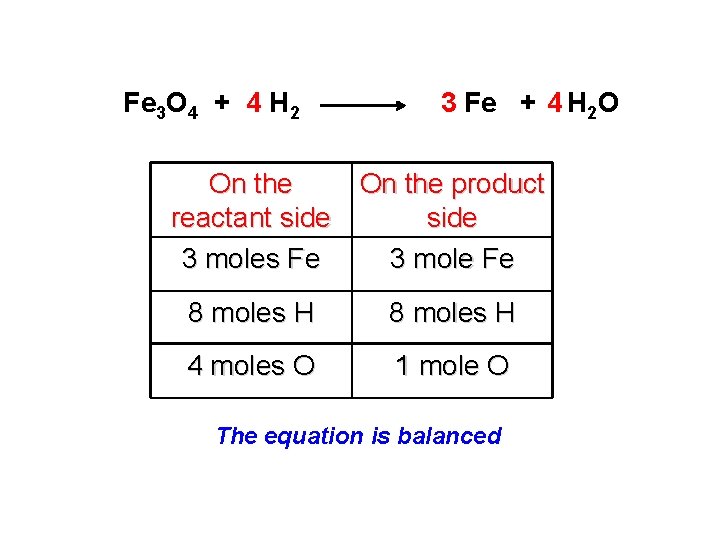
Fe 3 O 4 + H 2 Fe + H 2 O 1) Fe: Fe 3 O 4 + H 2 3 Fe + H 2 O 2) O: Fe 3 O 4 + H 2 3 Fe + 4 H 2 O 3) H: Fe 3 O 4 + 4 H 2 3 Fe + 4 H 2 O

Fe 3 O 4 + 4 H 2 3 Fe + 4 H 2 O On the product reactant side 3 moles Fe 3 mole Fe 8 moles H 4 moles O 1 mole O The equation is balanced