Part 1 CHAPTER 4 B THE ELECTRON STRUCTURE

Part 1 CHAPTER 4 B: THE ELECTRON STRUCTURE OF THE ATOM

Scientist realized every electron had it’s own location in the atom that depended on it’s energy Physicists developed an address system to keep track of who was where Quantum numbers- the solutions to various wave equations scientists use to describe energy, momentum, and probable location of an electron INTRODUCTION TO QUANTUM NUMBERS

Principal Quantum Number (n) Azimuthal Magnetic Quantum Number (l) Quantum Number (m) Electron-Spin Quantum Number (ms) 4 QUANTUM NUMBERS
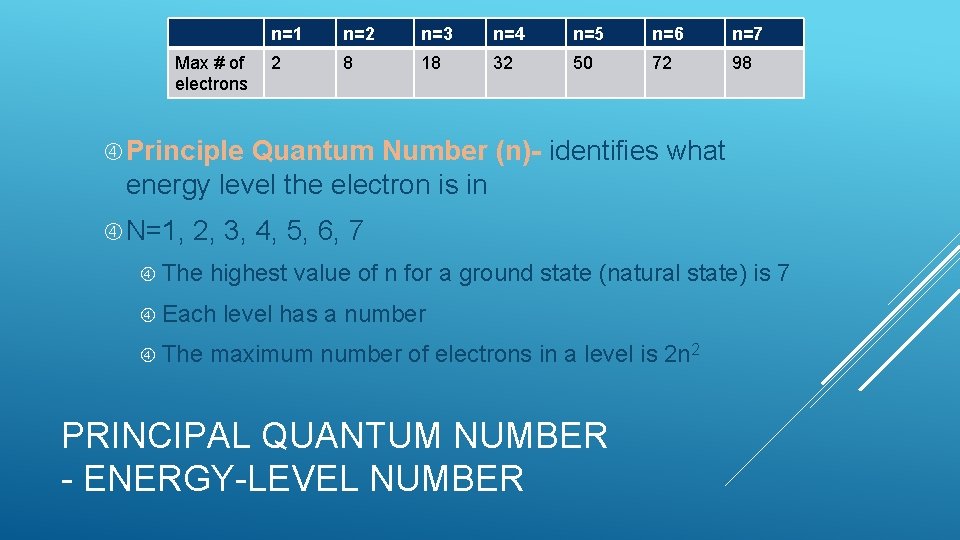
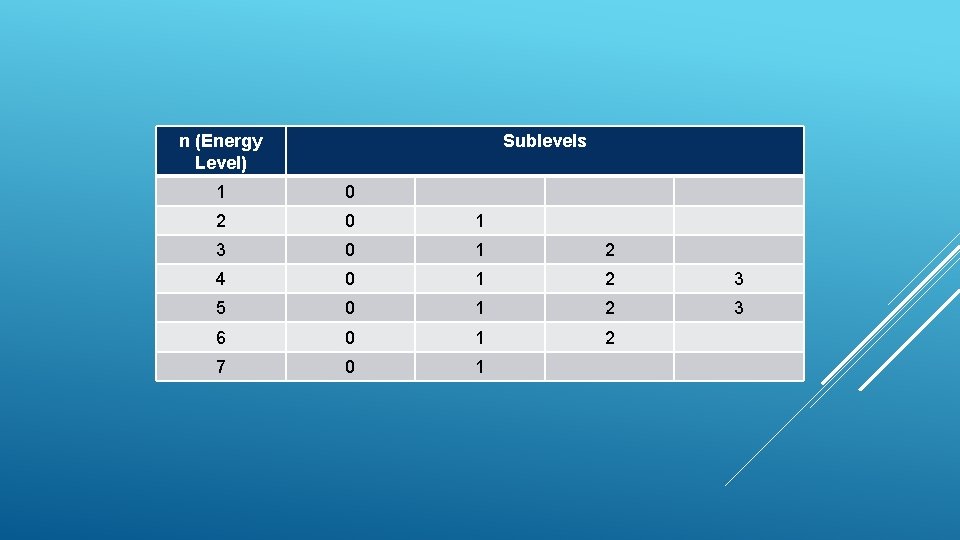
Max # of electrons n=1 n=2 n=3 n=4 n=5 n=6 n=7 2 8 18 32 50 72 98 Principle Quantum Number (n)- identifies what energy level the electron is in N=1, 2, 3, 4, 5, 6, 7 The highest value of n for a ground state (natural state) is 7 Each The level has a number maximum number of electrons in a level is 2 n 2 PRINCIPAL QUANTUM NUMBER - ENERGY-LEVEL NUMBER

Each energy level has sublevels. Each sublevel has a positive number called the azimuthal quantum number (l) Sublevels are between 0 and n-1 s= sublevel 0 P= sublevel 1 d= sublevel 2 f= sublevel 3 g= sublevel 4 h= sublevel 5 i= sublevel 6 NOTE: Sublevels 4, 5, 6 are only for EXCITED atoms (atoms with extra energy), not ground state atoms AZIMUTHAL QUANTUM NUMBERSUBLEVEL QUANTUM NUMBER
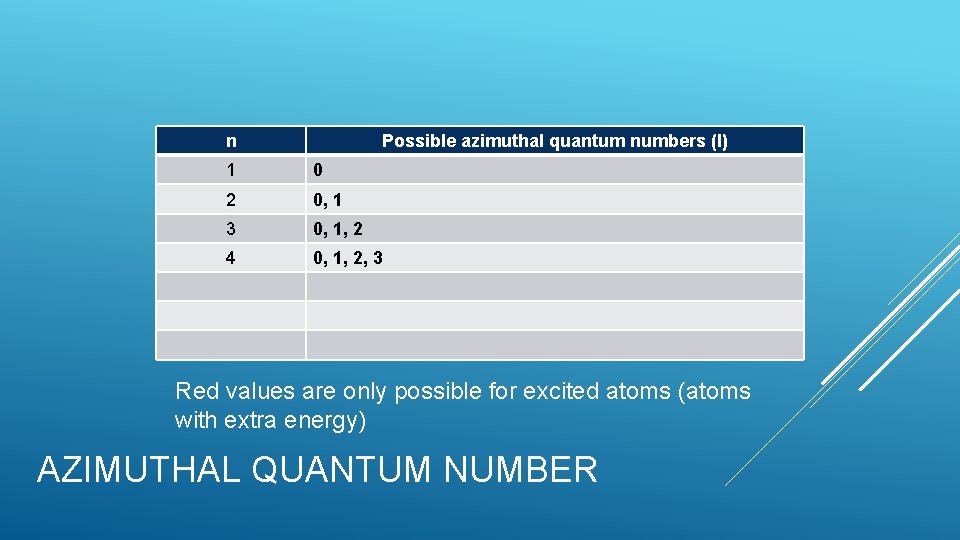
n Possible azimuthal quantum numbers (l) 1 0 2 0, 1 3 0, 1, 2 4 0, 1, 2, 3 Red values are only possible for excited atoms (atoms with extra energy) AZIMUTHAL QUANTUM NUMBER

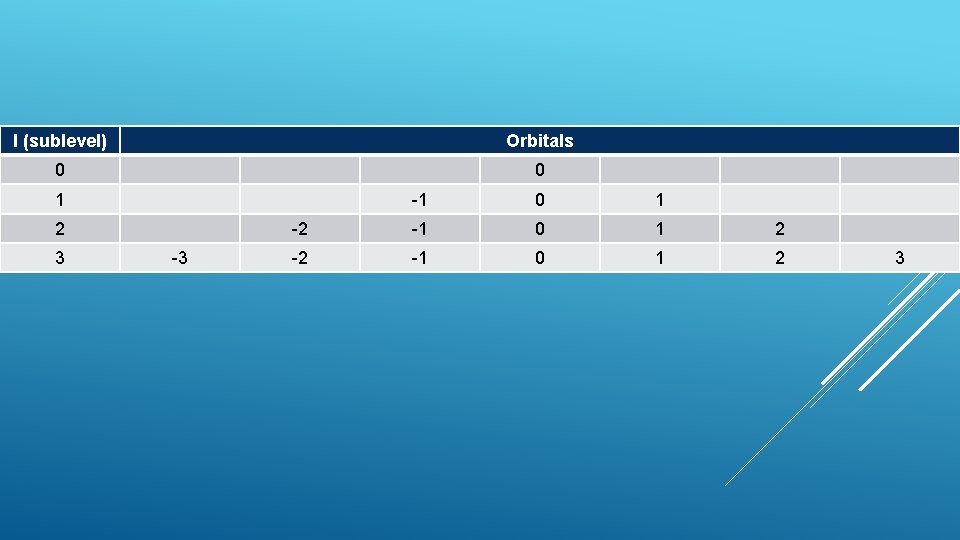
Each sublevel has different orbitals (m) How l =0 1 l orbital (m=0) =1 3 l many? 2 l + 1 orbitals (m= -1, 0, 1) =2 5 orbitals (m= -2, -1, 0, 1, 2) MAGNETIC QUANTUM NUMBERORBITAL QUANTUM NUMBER

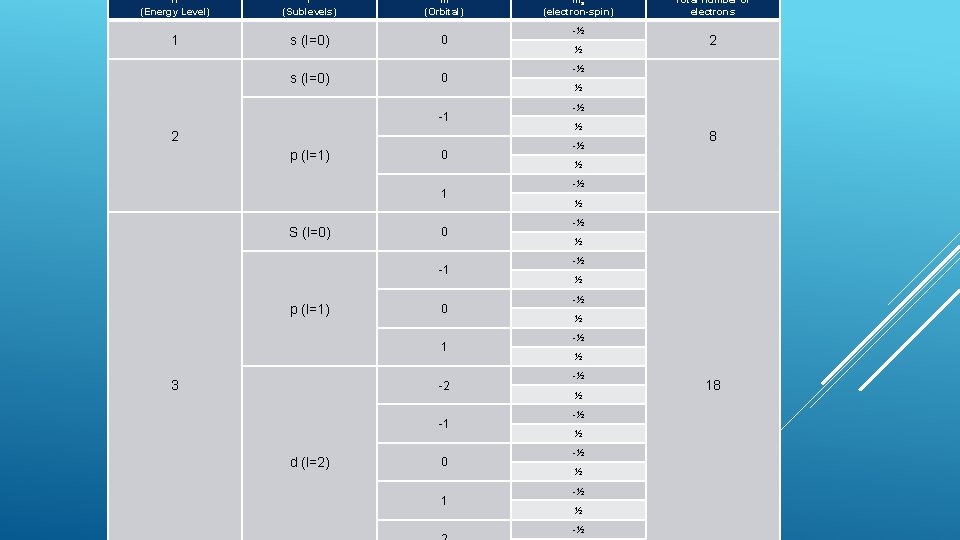
Since each sublevel (s, p, d, f, etc) has only 2 electrons Labeled with the electron-spin quantum number (ms), either -1/2 or +1/2 No two electrons in an atom can have the same set of four quantum numbers This is the Pauli Exclusion Principle PAULI EXCLUSION PRINCIPLE

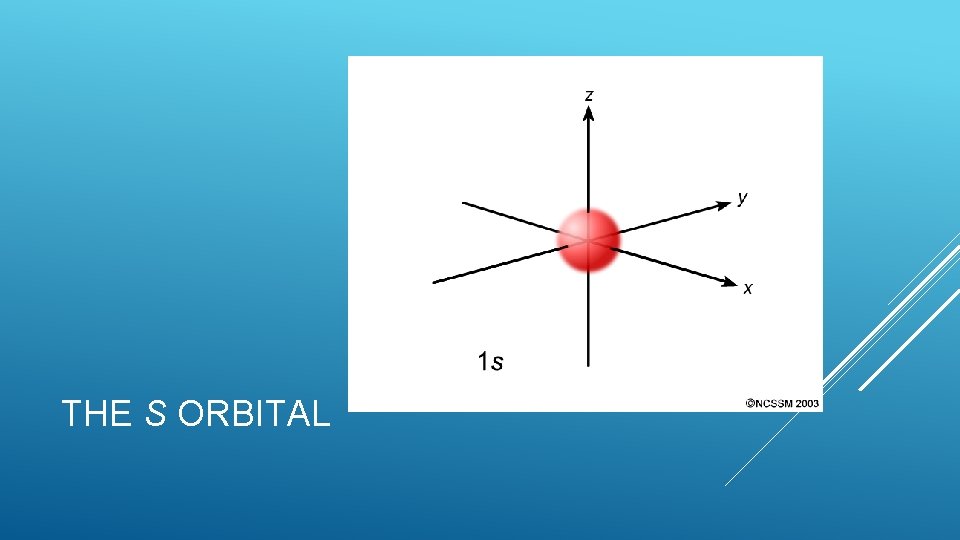
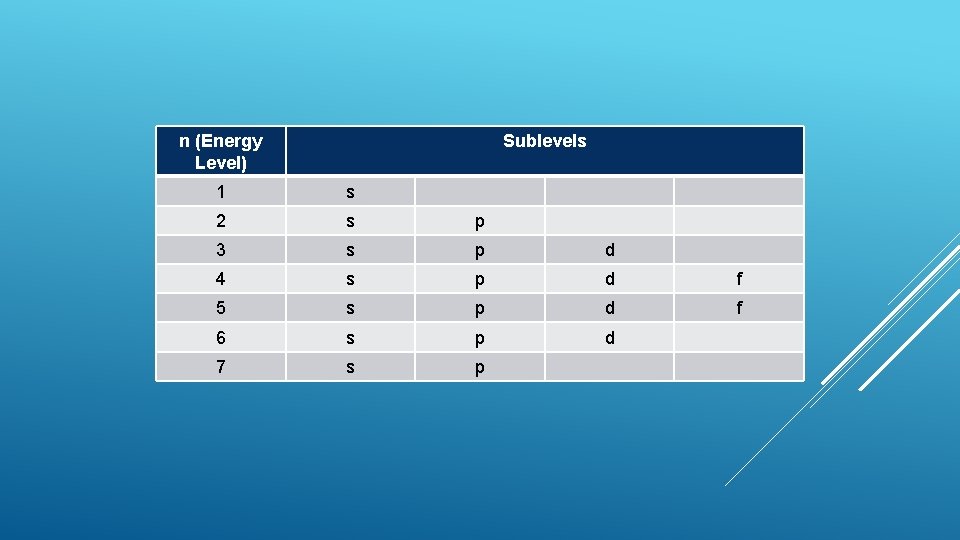
The s sublevel- Simplest Shaped Only like a circle 1 orbital (no suborbitals) Every energy level (n=1, 2, …. , 7) has an s sublevel Holds 2 electrons l =0 THE SUBLEVELS
THE S ORBITAL

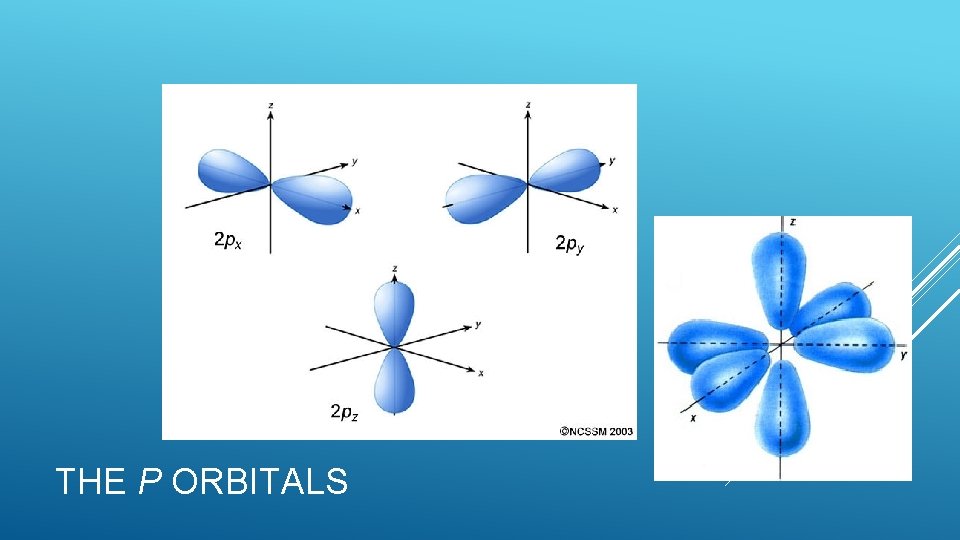
The p sublevel Has 3 orbitals Each shaped like a dumbell One on the x-axis, one on the y-axis, one on the z-axis Sometimes we say px, py, pz 2 electrons in each orbital One negative (-1/2), one positive (+1/2) Total l= electrons in p sublevel- 6 1 THE SUBLEVELS
THE P ORBITALS

The d sublevel More complicated There are 5 orbitals Each suborbital holds 2 electrons Total electrons in d sublevel- 10 l =2 THE SUBLEVELS
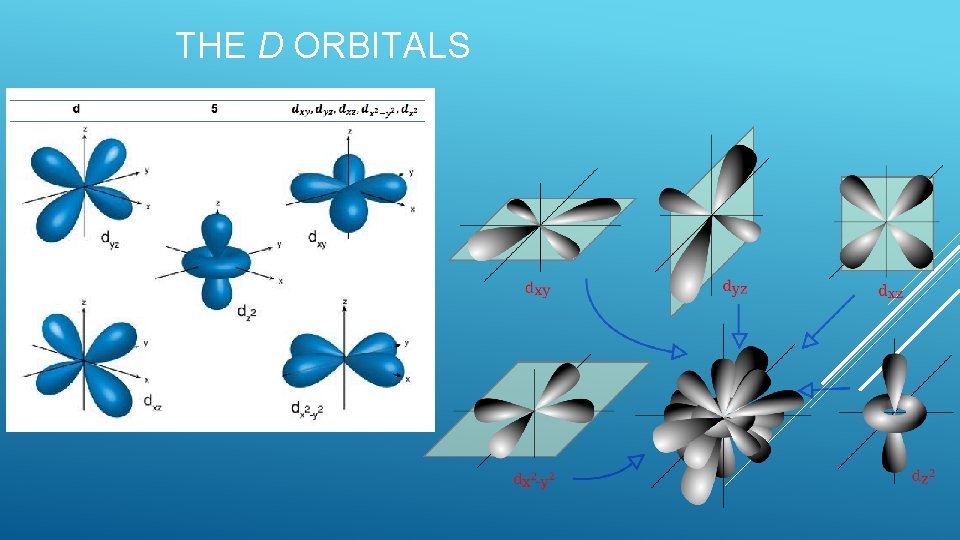
THE D ORBITALS

The f sublevel Even 7 possible orbitals Can l more complicated hold up to 14 electrons =3 THE SUBLEVELS

n (Energy Level) Sublevels 1 0 2 0 1 3 0 1 2 4 0 1 2 3 5 0 1 2 3 6 0 1 2 7 0 1
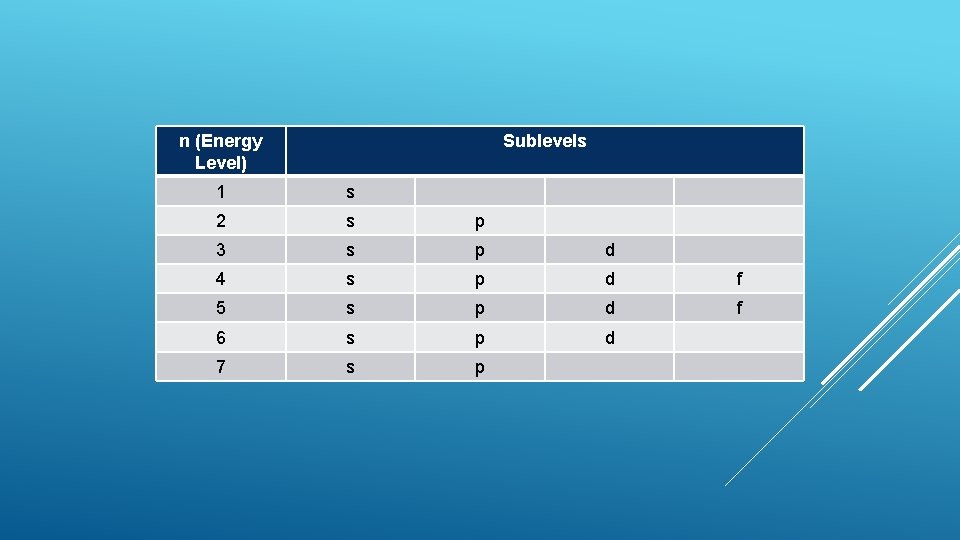
n (Energy Level) Sublevels 1 s 2 s p 3 s p d 4 s p d f 5 s p d f 6 s p d 7 s p
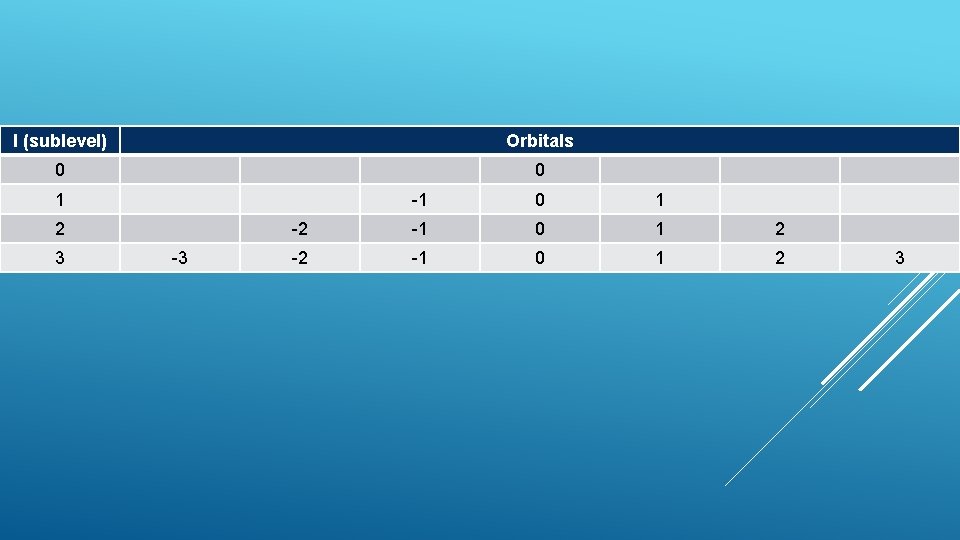
l (sublevel) Orbitals 0 0 1 2 3 -3 -1 0 1 -2 -1 0 1 2 3
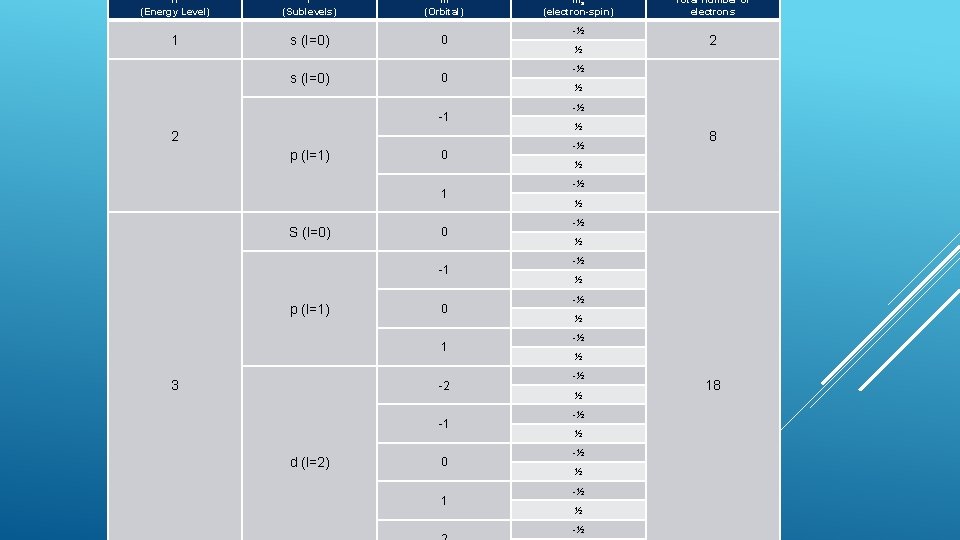
n (Energy Level) l (Sublevels) m (Orbital) 1 s (l=0) 0 -1 2 p (l=1) 0 1 S (l=0) 0 -1 p (l=1) 0 1 3 -2 -1 d (l=2) 0 1 ms (electron-spin) -½ ½ Total number of electrons 2 -½ ½ -½ 8 ½ -½ ½ -½ ½ -½ 18

Part 2 CHAPTER 4 B: THE ELECTRON STRUCTURE OF THE ATOM
n (Energy Level) Sublevels 1 0 2 0 1 3 0 1 2 4 0 1 2 3 5 0 1 2 3 6 0 1 2 7 0 1
n (Energy Level) Sublevels 1 s 2 s p 3 s p d 4 s p d f 5 s p d f 6 s p d 7 s p
l (sublevel) Orbitals 0 0 1 2 3 -3 -1 0 1 -2 -1 0 1 2 3
n (Energy Level) l (Sublevels) m (Orbital) 1 s (l=0) 0 -1 2 p (l=1) 0 1 S (l=0) 0 -1 p (l=1) 0 1 3 -2 -1 d (l=2) 0 1 ms (electron-spin) -½ ½ Total number of electrons 2 -½ ½ -½ 8 ½ -½ ½ -½ ½ -½ 18
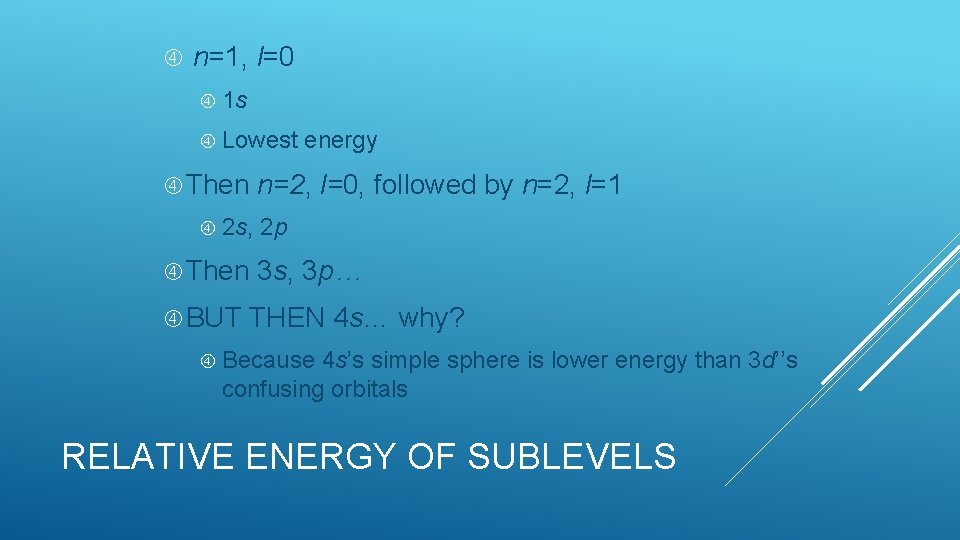
n=1, l=0 1 s Lowest Then 2 s, Then BUT energy n=2, l=0, followed by n=2, l=1 2 p 3 s, 3 p… THEN 4 s… why? Because 4 s’s simple sphere is lower energy than 3 d’’s confusing orbitals RELATIVE ENERGY OF SUBLEVELS
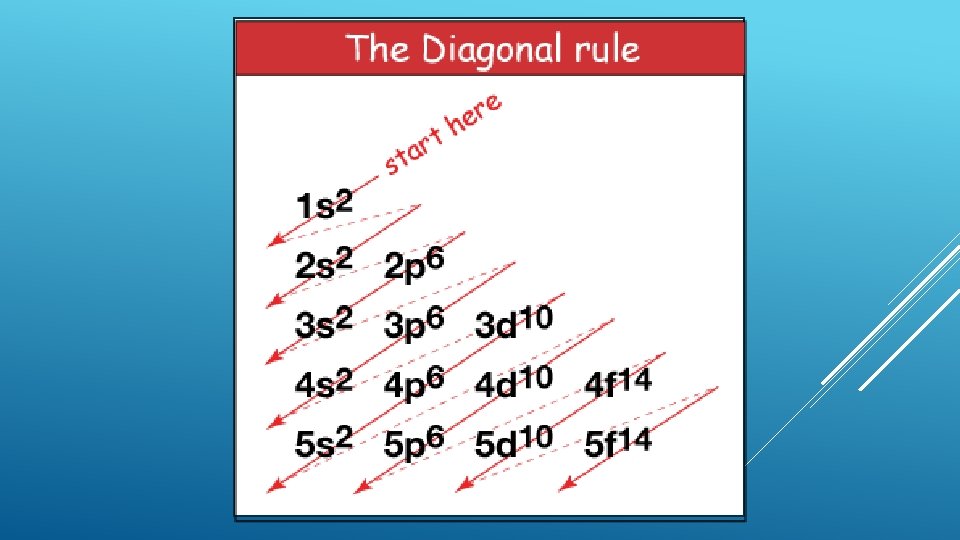
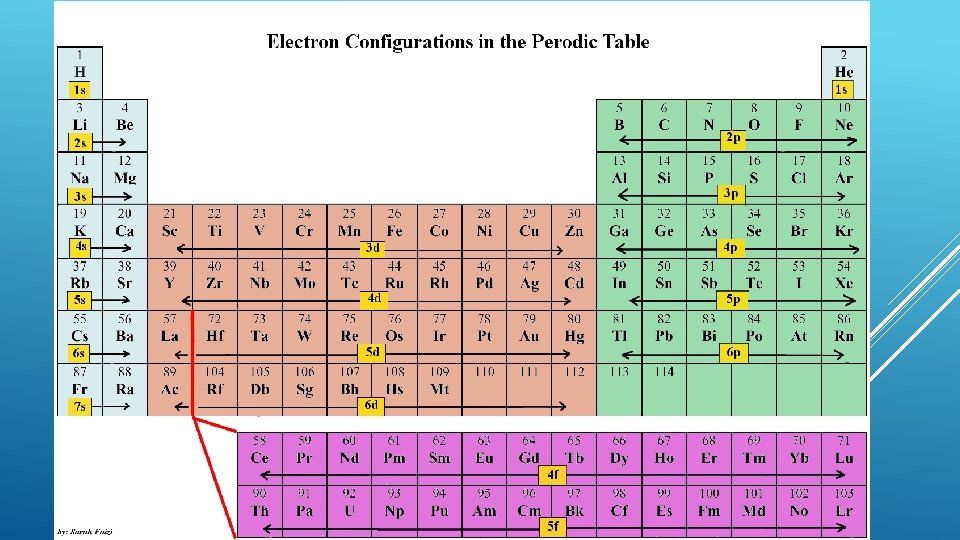

Aufbau principle- arrangement of electrons in an atom is made by adding electrons to an atom with fewer electrons Hydrogen has 1 electron 1 s sublevel Electron configuration- (arrangement of electrons) 1 s 1 Helium has 2 electrons 1 s sublevel Electron configuration- 1 s 2 ELECTRON CONFIGURATIONS

Special way to write this called orbital notation- illustrates electron configuration ORBITAL NOTATION

Hund’s rule- as electrons fill a sublevel, all orbitals receive one electron with the same spin before they begin to pair Like on the bus! ORBITAL NOTATION
- Slides: 31