Part 06 Proper Structure for Important Calculations Calculation


- Slides: 10

Part 06 Proper Structure for Important Calculations

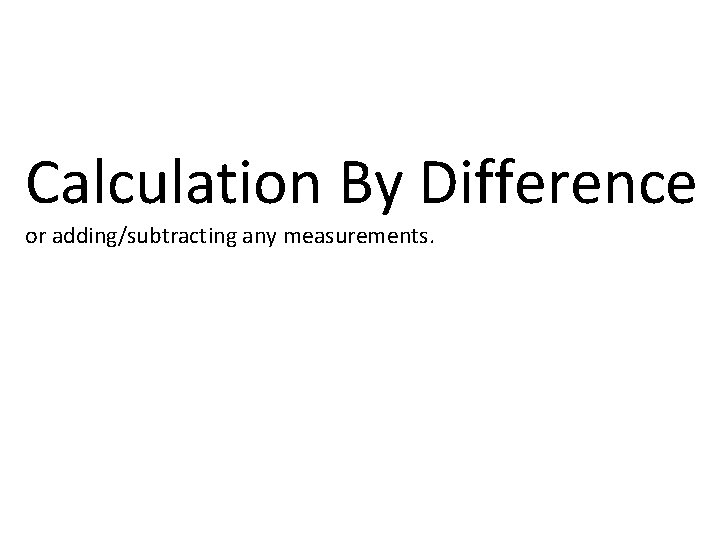
Calculation By Difference or adding/subtracting any measurements.

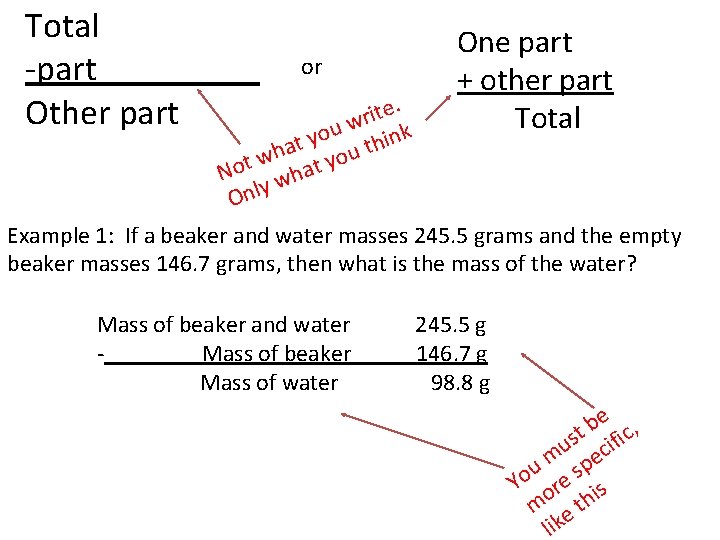
Total -part_____ Other part or One part + other part Total e. t i r w u o y ink t h t a h ou w y t t o N ha w y Onl Example 1: If a beaker and water masses 245. 5 grams and the empty beaker masses 146. 7 grams, then what is the mass of the water? Mass of beaker and water Mass of beaker Mass of water 245. 5 g 146. 7 g 98. 8 g e , b st ific u c m e u sp o Y ore is m e th lik

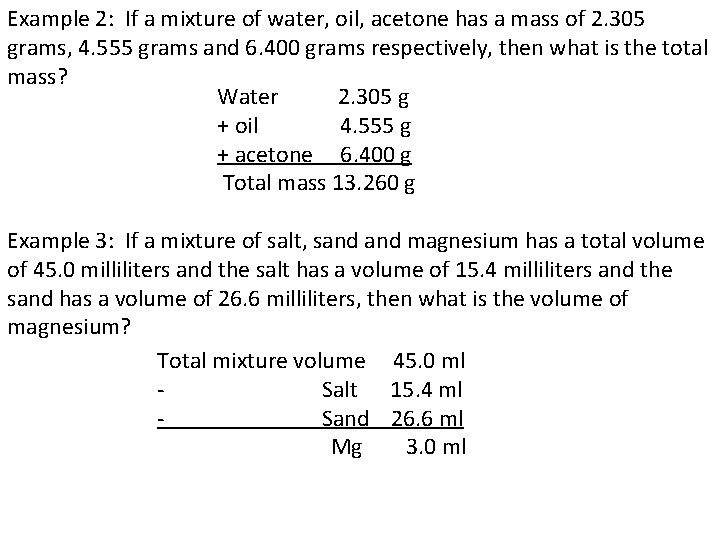
Example 2: If a mixture of water, oil, acetone has a mass of 2. 305 grams, 4. 555 grams and 6. 400 grams respectively, then what is the total mass? Water 2. 305 g + oil 4. 555 g + acetone 6. 400 g Total mass 13. 260 g Example 3: If a mixture of salt, sand magnesium has a total volume of 45. 0 milliliters and the salt has a volume of 15. 4 milliliters and the sand has a volume of 26. 6 milliliters, then what is the volume of magnesium? Total mixture volume 45. 0 ml Salt 15. 4 ml Sand 26. 6 ml Mg 3. 0 ml

Percents

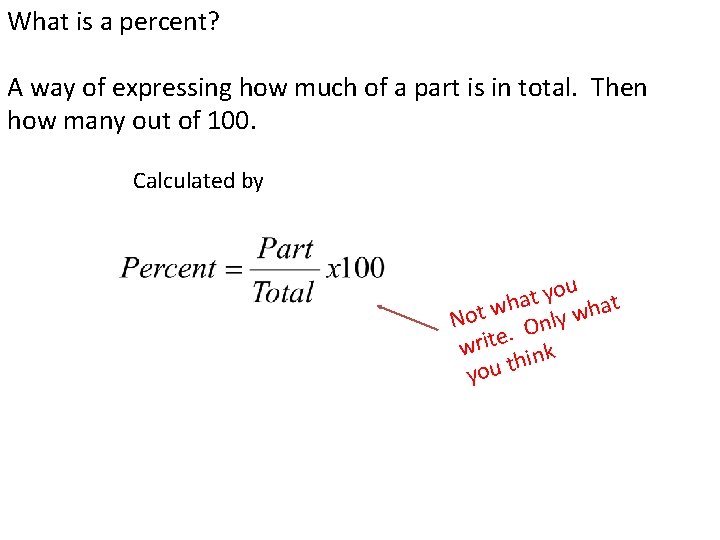
What is a percent? A way of expressing how much of a part is in total. Then how many out of 100. Calculated by ou y t a h at w h t w o N ly n O. write ink h you t

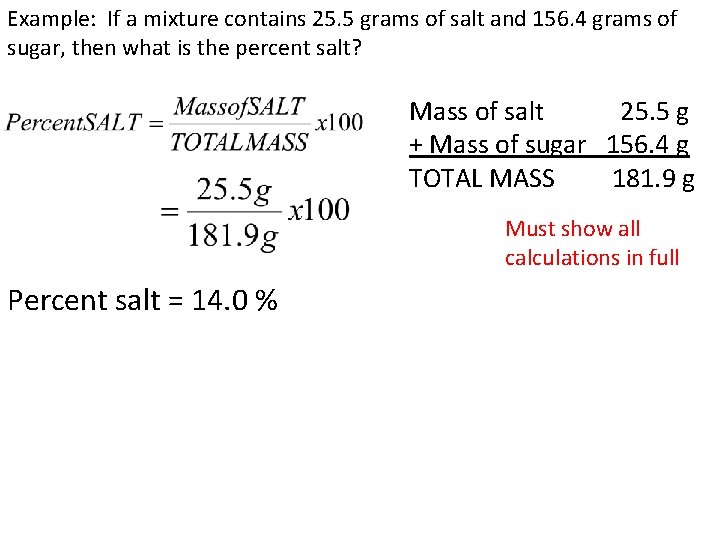
Example: If a mixture contains 25. 5 grams of salt and 156. 4 grams of sugar, then what is the percent salt? Mass of salt 25. 5 g + Mass of sugar 156. 4 g TOTAL MASS 181. 9 g Must show all calculations in full Percent salt = 14. 0 %
Averages

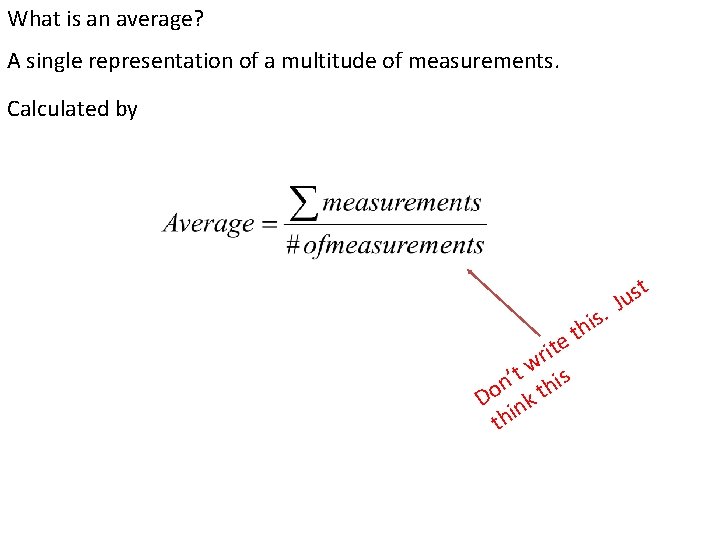
What is an average? A single representation of a multitude of measurements. Calculated by ite r w s t ’ n thi o D ink th . s i th t Jus

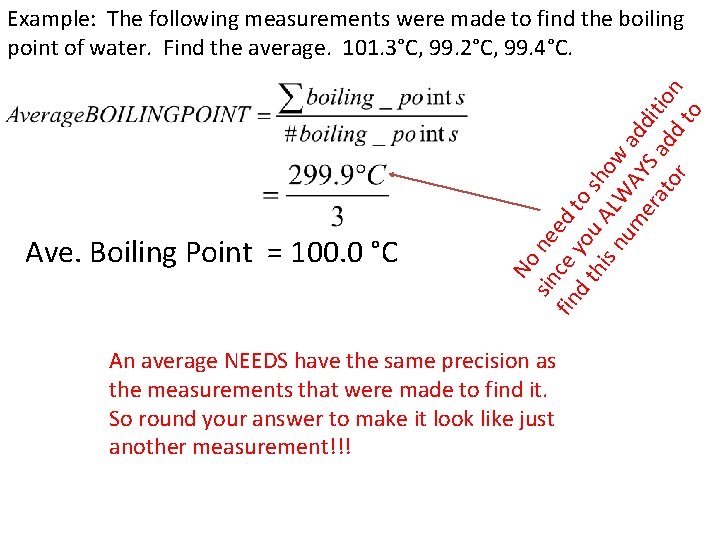
Ave. Boiling Point = 100. 0 °C No sin ne fin ce ed d t you to his A sho nu LW w a m AY dd er S at ad itio or d n to Example: The following measurements were made to find the boiling point of water. Find the average. 101. 3°C, 99. 2°C, 99. 4°C. An average NEEDS have the same precision as the measurements that were made to find it. So round your answer to make it look like just another measurement!!!