Part 05 Electronegativity and Bond Type 1 Types

Part 05 Electronegativity and Bond Type
1. Types of Covalent Bonds Covalent bonds fall into two categories depending on how the electrons are shared between the atoms.
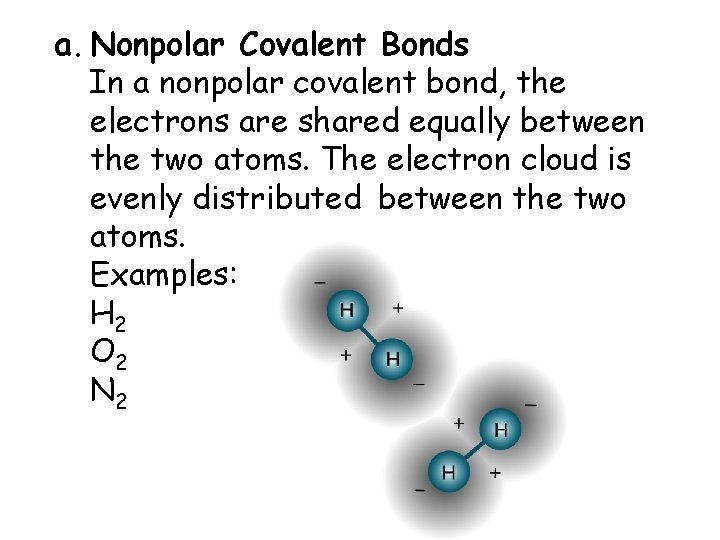
a. Nonpolar Covalent Bonds In a nonpolar covalent bond, the electrons are shared equally between the two atoms. The electron cloud is evenly distributed between the two atoms. Examples: H 2 O 2 N 2
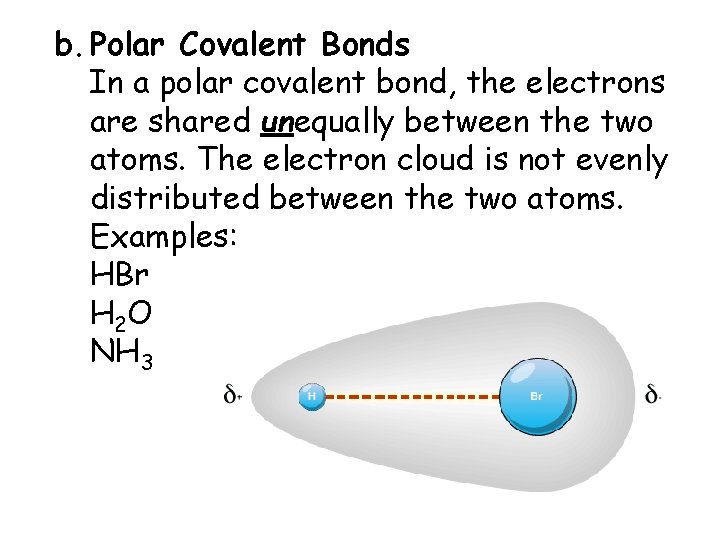
b. Polar Covalent Bonds In a polar covalent bond, the electrons are shared unequally between the two atoms. The electron cloud is not evenly distributed between the two atoms. Examples: HBr H 2 O NH 3
4. Determining the Type of Covalent Bond In order to determine the type of bond in a compound, electronegativity values are used. The difference in electronegativity values between two atoms determines the type of bond.
a. Electronegativity is a measure of an atom’s ability to attract bonded electrons. An atom’s electronegativity is quantified from 0. 0 to 4. 0. 1) Atoms with low EN values have weak attraction for bonded electrons. Example: Metals 2) Atoms with high EN values have strong attraction for bonded electrons. Example: The Halogens
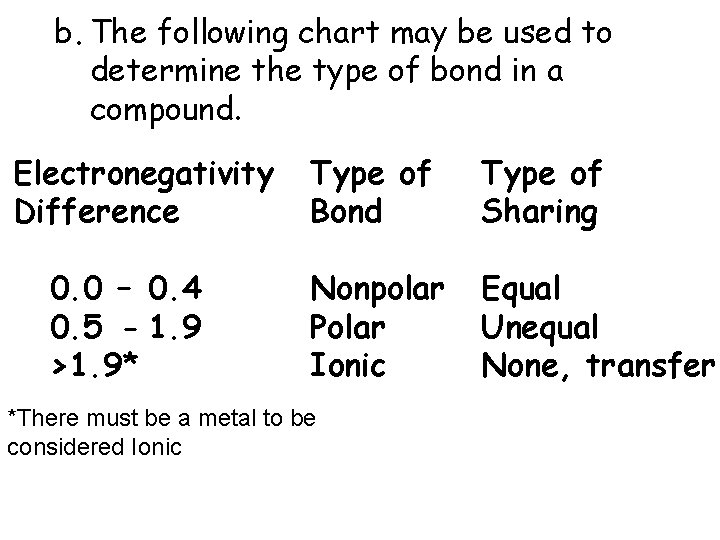
b. The following chart may be used to determine the type of bond in a compound. Electronegativity Difference 0. 0 – 0. 4 0. 5 - 1. 9 >1. 9* Type of Bond Type of Sharing Nonpolar Polar Ionic Equal Unequal None, transfer *There must be a metal to be considered Ionic
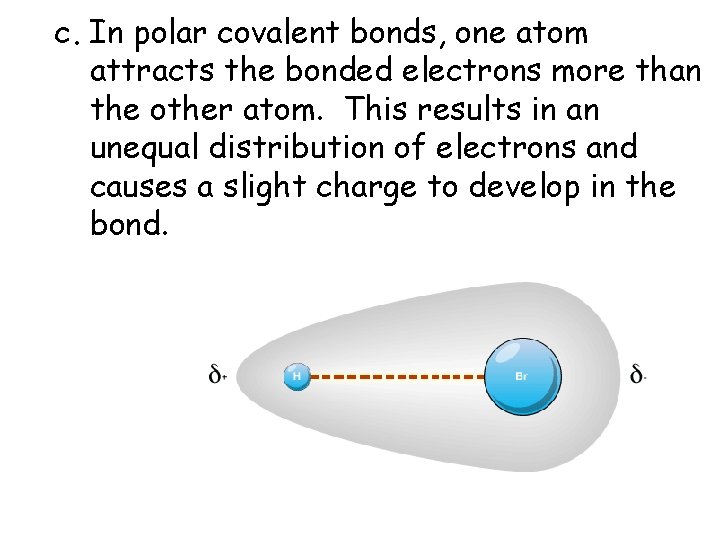
c. In polar covalent bonds, one atom attracts the bonded electrons more than the other atom. This results in an unequal distribution of electrons and causes a slight charge to develop in the bond.

c. The atom with the lower EN value will have the bonded electrons pulled away and that end of the bond will have a slightly positive charge. The symbol used for slightly positive is d+

c. The atom with the higher EN value will have the bonded electrons pulled toward it and that end of the bond will have a slightly negative charge. The symbol used for slightly negative is d-
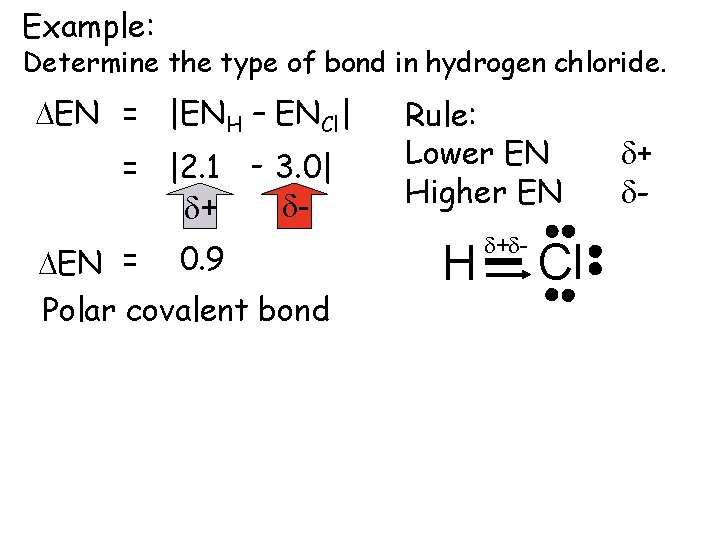
Example: Determine the type of bond in hydrogen chloride. DEN = |ENH – ENCl| = |2. 1 - 3. 0| dd+ DEN = 0. 9 Polar covalent bond Rule: Lower EN Higher EN H d+d- Cl d+ d-
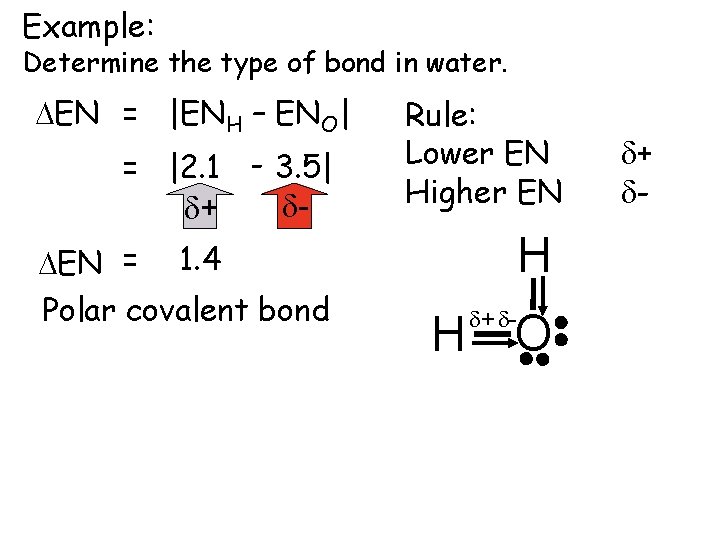
Example: Determine the type of bond in water. DEN = |ENH – ENO| = |2. 1 - 3. 5| dd+ DEN = 1. 4 Polar covalent bond Rule: Lower EN Higher EN H H d+ d- O d+ d-
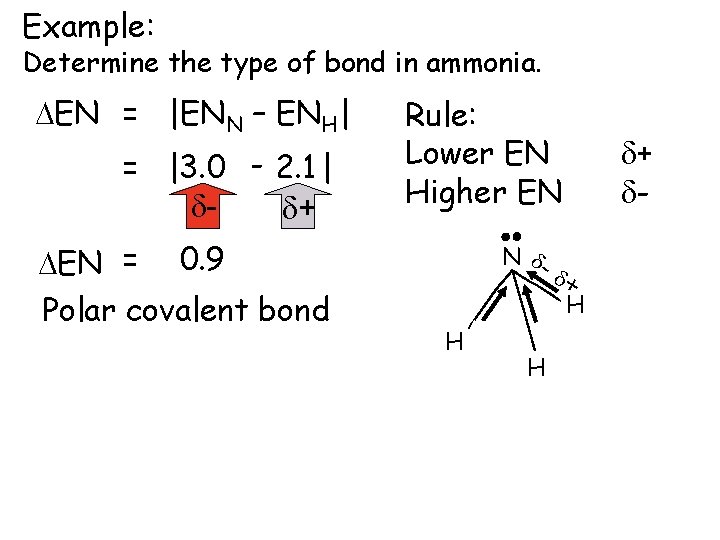
Example: Determine the type of bond in ammonia. DEN = |ENN – ENH| = |3. 0 - 2. 1| dd+ DEN = 0. 9 Polar covalent bond Rule: Lower EN Higher EN N dd+ H H H d+ d-
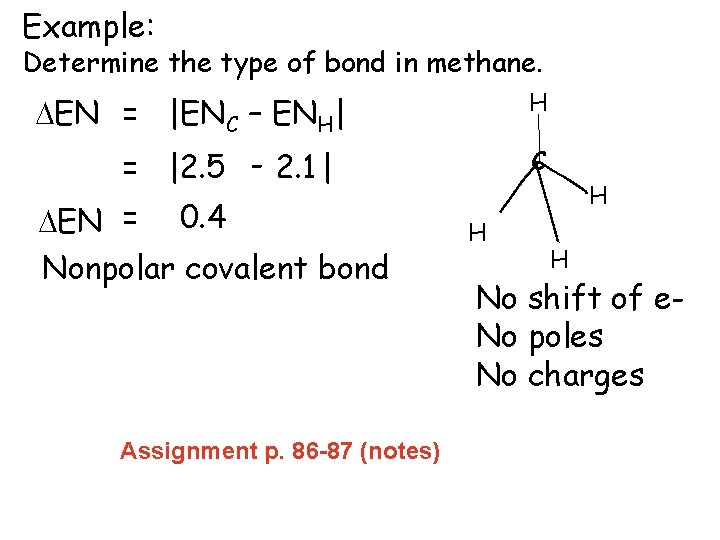
Example: Determine the type of bond in methane. H DEN = |ENC – ENH| = |2. 5 - 2. 1| DEN = 0. 4 Nonpolar covalent bond Assignment p. 86 -87 (notes) C H H H No shift of e. No poles No charges
- Slides: 14