PARENTERALS QUALITY CONTROL TESTS DEPARTMENT OF PHARMACEUTICS CHALAPATHI











- Slides: 23

PARENTERALS QUALITY CONTROL TESTS DEPARTMENT OF PHARMACEUTICS CHALAPATHI INSTITUTE OF PHARMACEUTICAL SCIENCES

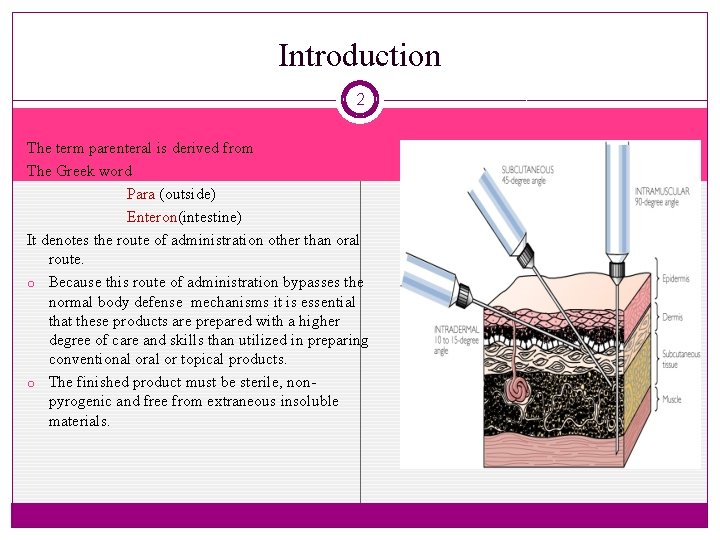
Introduction 2 The term parenteral is derived from The Greek word Para (outside) Enteron(intestine) It denotes the route of administration other than oral route. o Because this route of administration bypasses the normal body defense mechanisms it is essential that these products are prepared with a higher degree of care and skills than utilized in preparing conventional or topical products. o The finished product must be sterile, nonpyrogenic and free from extraneous insoluble materials.

Quality Control tests for Parenterals 3 � Quality control is a concept which strives to produce a perfect product by a series of measures designed to prevent and eliminate errors at different stages of production. � There are mainly four Quality control tests performed. They are i. leakage test ii. Pyrogen test iii. Sterility test iv. Particulate evaluation (clarity test)

Leakage test 4 � This test is performed only for ampoules which have been sealed by fusion to ensure that there should not be any leakage in them. � Vials & bottles not subjected to this test because of flexibility of rubber. � There are two methods for the identification of leaks in parenterals they are 1. Methylene blue dye test 2. spark test

Methylene blue dye test 5 � The ampoules are immersed in vacuum chamber consisting of 1% methylene blue solution � A vacuum of about 27 inch Hg is created for about 15 to 30 min. � This causes the solution to enter the ampoules with defective sealing. � The vacuum is released and ampoules are observed. � If a leakage is present, the solution in the ampoules appear blue color.

Spark test 6 � The machine uses high precision electrodes to inspect the full circumference of the containers, including the closure zone. � All containers are presented individually to the electrodes. � Any moisture that has penetrated through capillary forces in a crack, pinhole or just weak glass is registered as a change in resistance. � Appearance of blue color confirms the presence of leakage.

Sterilty test 7 � The test is designed to identify the presence of viable forms of bacteria , fungi and yeasts in substances , preparations or articles which are required to be sterile. � The primary official test is performed by means of filteration , but direct transfer is used for membrane filteration when unsuitable. � Methods of testing: a. Membrane filtration method b. Direct inoculation method media used Fluid thioglycollate medium Soya bean casein digest medium

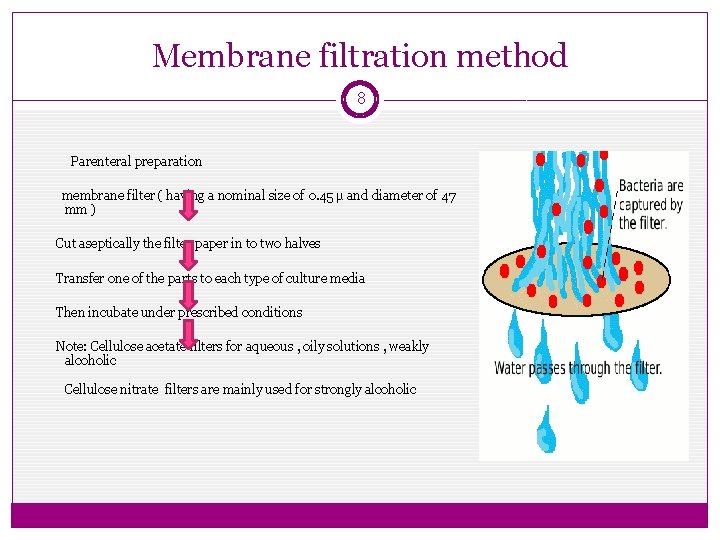
Membrane filtration method 8 Parenteral preparation membrane filter ( having a nominal size of 0. 45 µ and diameter of 47 mm ) Cut aseptically the filter paper in to two halves Transfer one of the parts to each type of culture media Then incubate under prescribed conditions Note: Cellulose acetate filters for aqueous , oily solutions , weakly alcoholic Cellulose nitrate filters are mainly used for strongly alcoholic

Direct inoculation method 9 Parenteral preparation Inoculate to culture media Incubate at prescribed conditions for 14 days No growth then the test is positive suitable method for aqueous solutions, oily liquids, ointments and creams

Pyrogen test 10 � Pyrogens are Produced mostly by gram-negative bacteria. � Pyrogens are fever producing metabolic products of micro organisms � The presence of pyrogens in parenteral preperations is evaluated by a qualitative fever response test in rabbits. � Test for pyrogens can be carried out by in-vitro and in-vivo methods. A) Rabbit test (in-vivo) B) LAL test (Limulus amoebocyte lysate) (in-vitro)

Rabbit test 11 � PRINCIPLE: The test involves measurement of rise in body temperature of the rabbits following the intravenous injection of a sterile solution of the substance to be tested. The body temperature of the rabbits increases if pyrogens are present in the injected test solution. � SELECTION OF TEST ANIMALS: Healthy , adult rabbits of either sex, each weighing not less than 1. 5 kg. Do not use any rabbit for main test if: 1) It shows a temperature variation greater than 0. 20 c between two successive readings noted during the determination of initial temperature. 2) And it’s temperature is higher than 39. 80 c or lower than 380 c.

12 � EQUIPMENTS REQUIRED FOR THE TEST: All glass ware, syringes, needles and thermometer must be thoroughly washed with water for injection and heated in a hot air oven at 2500 c for 30 minutes or at 2000 c for 1 hr. Retaining boxes for rabbits. TEST: The test is carried out on a group of three rabbits.

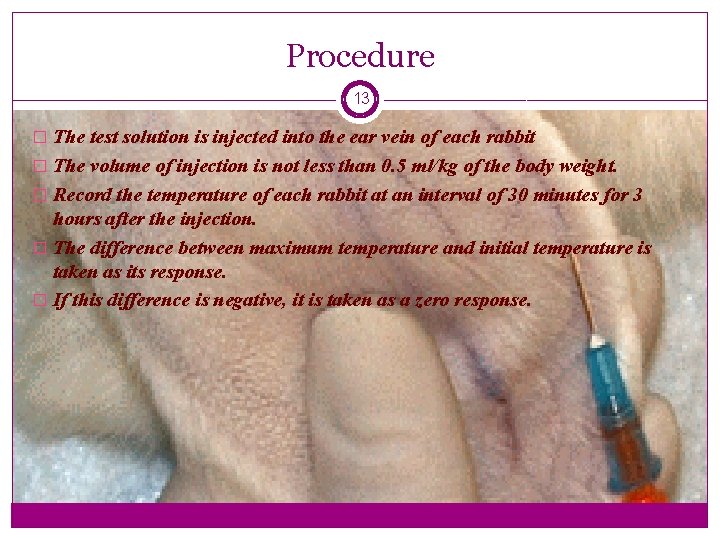
Procedure 13 � The test solution is injected into the ear vein of each rabbit � The volume of injection is not less than 0. 5 ml/kg of the body weight. � Record the temperature of each rabbit at an interval of 30 minutes for 3 hours after the injection. � The difference between maximum temperature and initial temperature is taken as its response. � If this difference is negative, it is taken as a zero response.

Interpretation of results 14 � Interpretation of results: If the response of any individual rabbit is less than 0. 60 c and if the some of the responses of the 3 rabbits is less than 1. 40 c, the preparation being examined passes the test. If the response of any rabbit is 0. 60 c or more or if the sum of the responses of the 3 rabbits is more than 1. 40 c, then the same test is repeated on another 5 rabbits. If not more than 3 of the 8 rabbits show individual responses of 0. 60 c or more and if the some of the responses of the 8 rabbits is not more than 3. 70 c, the preparation being examined passes the test.

LAL ( BET) test 15 Bacterial endotoxin test(BET) is an invitro test based on the formation of gel or the development of color in the presence of the amoebocytes of horseshoe crab ( Limulus polyphemus). Principle: The addition of a test solution containing of endotoxin (if present) to a solution of lysate produce turbidity or precipitation or gel formation of mixture. The rate of reaction depends on the concentration of the endotoxin. Types : 1) The gel clot test 2) The turbidimetric test 3) The kinetic chromogenic test

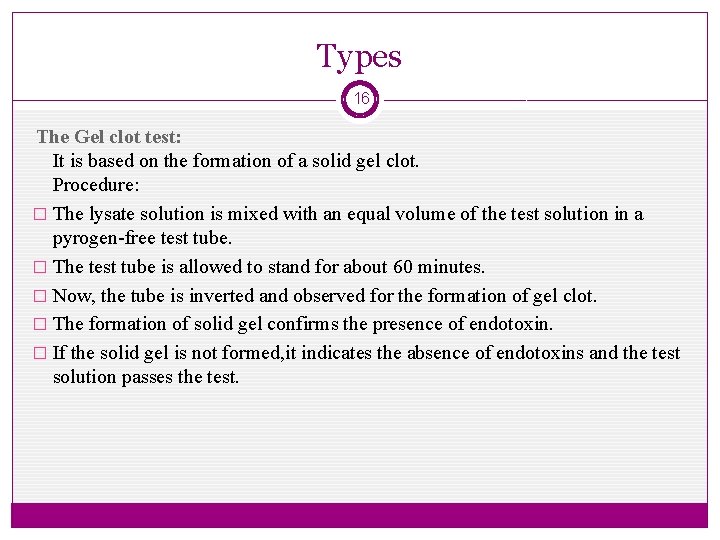
Types 16 The Gel clot test: It is based on the formation of a solid gel clot. Procedure: � The lysate solution is mixed with an equal volume of the test solution in a pyrogen-free test tube. � The test tube is allowed to stand for about 60 minutes. � Now, the tube is inverted and observed for the formation of gel clot. � The formation of solid gel confirms the presence of endotoxin. � If the solid gel is not formed, it indicates the absence of endotoxins and the test solution passes the test.

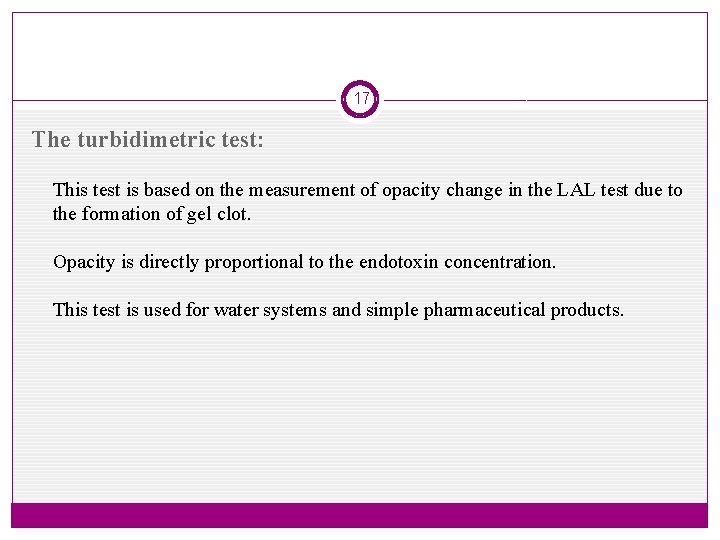
17 The turbidimetric test: This test is based on the measurement of opacity change in the LAL test due to the formation of gel clot. Opacity is directly proportional to the endotoxin concentration. This test is used for water systems and simple pharmaceutical products.

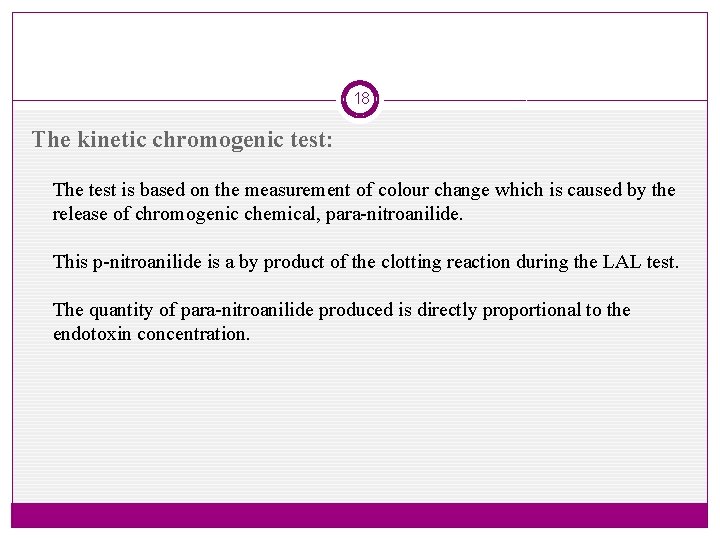
18 The kinetic chromogenic test: The test is based on the measurement of colour change which is caused by the release of chromogenic chemical, para-nitroanilide. This p-nitroanilide is a by product of the clotting reaction during the LAL test. The quantity of para-nitroanilide produced is directly proportional to the endotoxin concentration.

Procedure 19 � Into each test tubes dispense a volume appropriate to chosen receptacle of positive control, negative control and test solution � Add to each test tube equal volume of the appropriately constituted lysate. � Place and incubate at 37± 10 c for 1 hr. Interpretation of results � The preparation or substance being examined complies with pyrogen test if result of the positive product control is positive and negative product control is negative. � LAL test is cheaper, quicker and more accurate than other tests.

Clarity test 20 � Particulate matter can be detected in parenteral product by two methods, 1. i. ii. 2. i. ii. including visual inspection and electronic particulate counting. Visual methods Visual inspection by naked eye Automated visual inspection Particle count method Optical microscopic method Automated particle counter

Visual methods 21 i. Visual inspection by naked eye: In visual inspection, each injectable is inspected visually against white and black backgrounds. The white background aids in detection of dark colored particles. The light or reflective particles will appear against the black back ground. ii. Automated visual inspection: The automatic systems evaluates the particles in injectables automatically. However, this method requires destruction of the ampoule/container for the particle examination.

Particle count method 22 i. Microscopic count method: Membrane filters and microscopes are used. ii. Automated particle counter s: The automated particle counters are based on the light obscuration, light scattering method and the electrical resistance methods. Coulter Counter counts the particles in a sample based on the change in the electrical resistance. Particle size detection limit in this instrument is from 0. 1 to 1000 micrometer.

References 23 REFERENCES • United States Pharmacopoeia , Appendix 788, 56. • Indian Pharmacopoeia page no: 659 to 660 • Remington, The science and Practice of Pharmacy, 21 st ed. Page no. 1367 to 1374 • Michael J. Akers and Daniel S. Larrimore, Parenteral Quality Control. • rxpharmainfo. blogspot. com/. . . /in-process-and-finished-product-qc. • memberfiles. freewebs. com/95/47/65154795/. . . /6. PARENTERAL. pdf • pharmacyminds. blogspot. com/. . . /parenteral-quality-control
 Quality control test for parenteral
Quality control test for parenteral N chalapathi
N chalapathi Kronigs isthmus
Kronigs isthmus Tablets quality control tests
Tablets quality control tests Ace different tests help iq still
Ace different tests help iq still Cutting quality control
Cutting quality control Quality assurance vs quality control
Quality assurance vs quality control Quality control vs quality assurance pmp
Quality control vs quality assurance pmp Pmbok quality management
Pmbok quality management Quality control concepts
Quality control concepts Turbidity apes
Turbidity apes Antioxidants used in parenterals
Antioxidants used in parenterals Large volume parenterals contain
Large volume parenterals contain Clarity test for parenterals
Clarity test for parenterals Size separation process
Size separation process Granul effervescent
Granul effervescent The galenicals prepared by extraction of
The galenicals prepared by extraction of In situ soap method
In situ soap method How powders containing potent drugs are dispensed
How powders containing potent drugs are dispensed Ideal properties of suppository bases
Ideal properties of suppository bases Donor acceptor ratio in physical pharmacy
Donor acceptor ratio in physical pharmacy Importance of surface tension in pharmacy
Importance of surface tension in pharmacy Pharmaceutical incompatibility classification
Pharmaceutical incompatibility classification What is tolerated incompatibility
What is tolerated incompatibility