Pain Perception its Therapeutic Management PAIN 1 Physiologic













- Slides: 31

Pain Perception & its Therapeutic Management

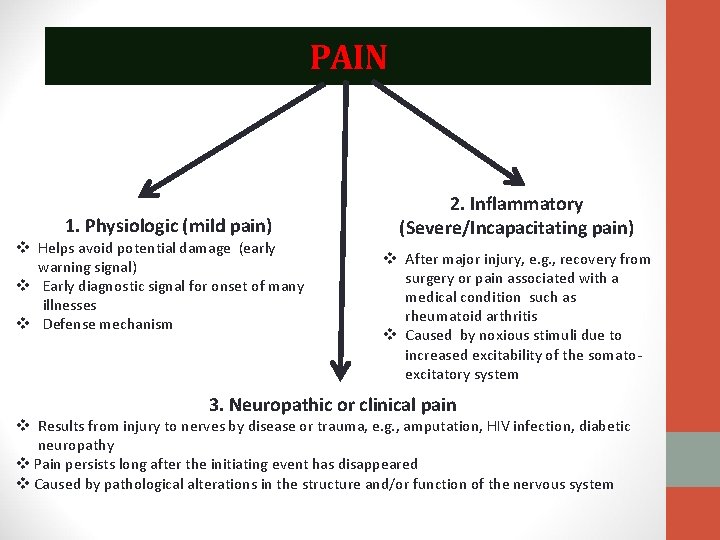
PAIN 1. Physiologic (mild pain) v Helps avoid potential damage (early warning signal) v Early diagnostic signal for onset of many illnesses v Defense mechanism 2. Inflammatory (Severe/Incapacitating pain) v After major injury, e. g. , recovery from surgery or pain associated with a medical condition such as rheumatoid arthritis v Caused by noxious stimuli due to increased excitability of the somatoexcitatory system 3. Neuropathic or clinical pain v Results from injury to nerves by disease or trauma, e. g. , amputation, HIV infection, diabetic neuropathy v Pain persists long after the initiating event has disappeared v Caused by pathological alterations in the structure and/or function of the nervous system

Pathophysiology of pain • Pain is an ill-defined, unpleasant sensation, evoked by an external or internal noxious stimulus. • Tissue damage activates free nerve endings (nociceptors) of peripheral nerves • Pain signal is transmitted to the spinal cord, hypothalamus, and cerebral cortex • Pain is transmitted to spinal cord by Adelta fibers and C fibers. • A-delta fibers transmit fast, sharp, welllocalized pain signals. C fibers conduct the pain signal slowly and produce poorly localized, dull, or burning type of pain. • Thalamus is the ‘relay’ station for incoming stimuli, including pain. It receives pain signals, processes it and forward it to the cerebral cortex

Pain fibers and pathways • A-delta (Aδ) fibers found in the skin and muscle, myelinated, respond to mechanical stimuli. They produce intermittent pain. • C fibers distributed in the muscle as well as the periosteum and the viscera. These fibers are unmyelinated, conduct thermal, chemical and strong mechanical stimuli. They produce persistent pain.

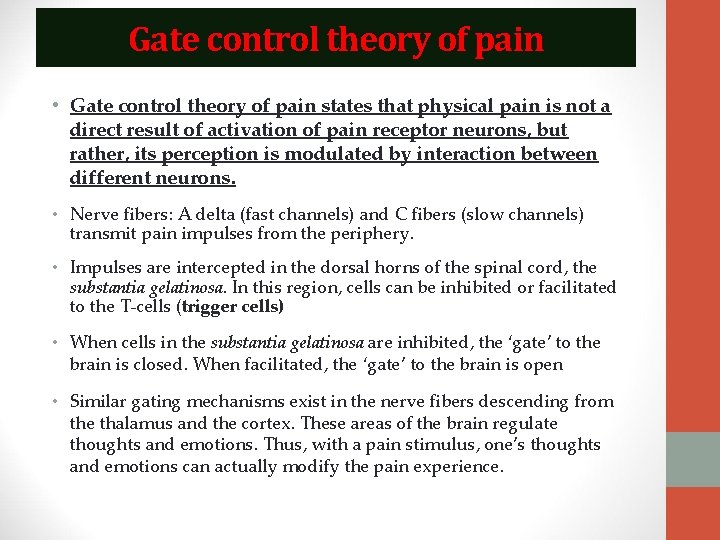
Gate control theory of pain • Gate control theory of pain states that physical pain is not a direct result of activation of pain receptor neurons, but rather, its perception is modulated by interaction between different neurons. • Nerve fibers: A delta (fast channels) and C fibers (slow channels) transmit pain impulses from the periphery. • Impulses are intercepted in the dorsal horns of the spinal cord, the substantia gelatinosa. In this region, cells can be inhibited or facilitated to the T-cells (trigger cells) • When cells in the substantia gelatinosa are inhibited, the ‘gate’ to the brain is closed. When facilitated, the ‘gate’ to the brain is open • Similar gating mechanisms exist in the nerve fibers descending from the thalamus and the cortex. These areas of the brain regulate thoughts and emotions. Thus, with a pain stimulus, one’s thoughts and emotions can actually modify the pain experience.

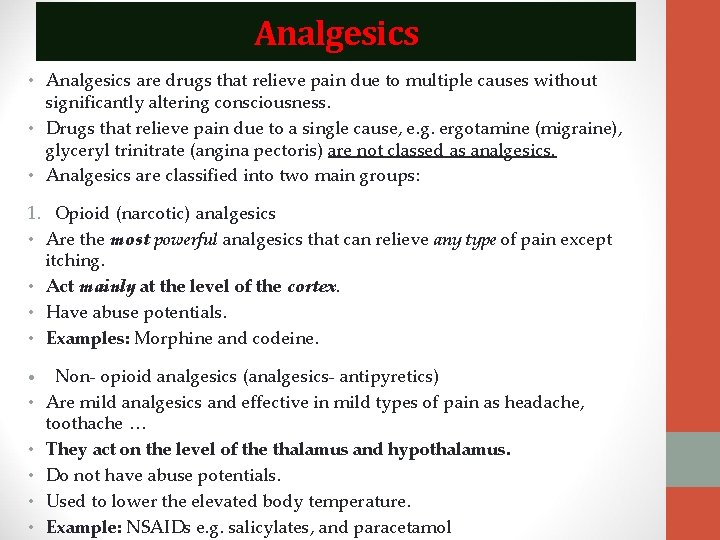
Analgesics • Analgesics are drugs that relieve pain due to multiple causes without significantly altering consciousness. • Drugs that relieve pain due to a single cause, e. g. ergotamine (migraine), glyceryl trinitrate (angina pectoris) are not classed as analgesics. • Analgesics are classified into two main groups: 1. Opioid (narcotic) analgesics • Are the most powerful analgesics that can relieve any type of pain except itching. • Act mainly at the level of the cortex. • Have abuse potentials. • Examples: Morphine and codeine. • Non- opioid analgesics (analgesics- antipyretics) • Are mild analgesics and effective in mild types of pain as headache, toothache … • They act on the level of the thalamus and hypothalamus. • Do not have abuse potentials. • Used to lower the elevated body temperature. • Example: NSAIDs e. g. salicylates, and paracetamol

Opioids • Opioids: Compound with morphine-like activity that interact with opioid receptors. • Opium, the source of morphine, is obtained from the poppy, Papaver somniferum and P album. • Opium contains many alkaloids, the principal one being morphine, which is present in a concentration of about 10%. Codeine can also be found in opium and is synthesized commercially from morphine. • The term opioid describes all compounds that work at opioid receptors. The term opiate specifically describes the naturally occurring alkaloids: morphine, codeine, thebaine, and papaverine. • They relieve moderate to severe pain by inhibiting release of Substance P in central and peripheral nerves; reducing the perception of pain sensation in brain, producing sedation and decreasing emotional upsets associated with pain

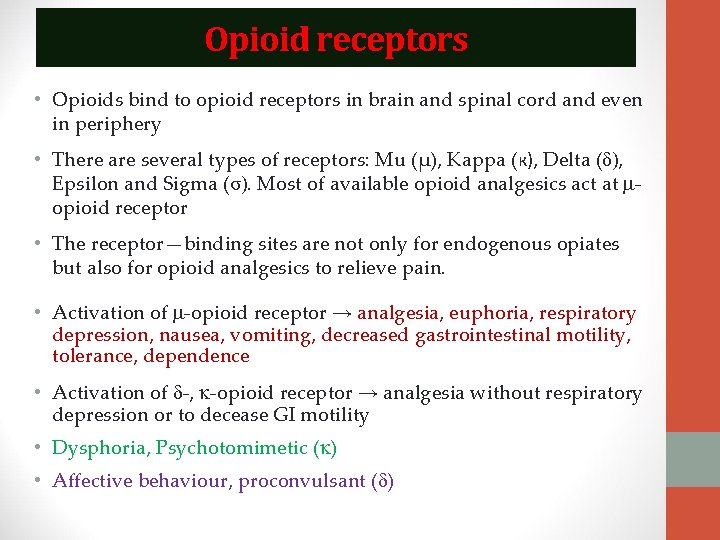
Opioid receptors • Opioids bind to opioid receptors in brain and spinal cord and even in periphery • There are several types of receptors: Mu (µ), Kappa (к), Delta (δ), Epsilon and Sigma (σ). Most of available opioid analgesics act at opioid receptor • The receptor—binding sites are not only for endogenous opiates but also for opioid analgesics to relieve pain. • Activation of -opioid receptor → analgesia, euphoria, respiratory depression, nausea, vomiting, decreased gastrointestinal motility, tolerance, dependence • Activation of -, -opioid receptor → analgesia without respiratory depression or to decease GI motility • Dysphoria, Psychotomimetic ( ) • Affective behaviour, proconvulsant ( )

Endogenous opioid peptides • Opioid alkaloids (e. g. , morphine) produce analgesia through actions at central nervous system (CNS) receptors that also respond to certain endogenous peptides with opioid-like pharmacologic properties. • The general term currently used for these endogenous substances is endogenous opioid peptides. • A number of endogenous opioid peptides having morphine like activity are found in brain, pituitary, spinal cord and GIT. They are: 1. 2. 3. 4. 5. β-Endorphins - Enkephalins - & Dynorphins- Endomorphins- Nociceptin- NOP receptor (nociceptin opioid peptide receptor)

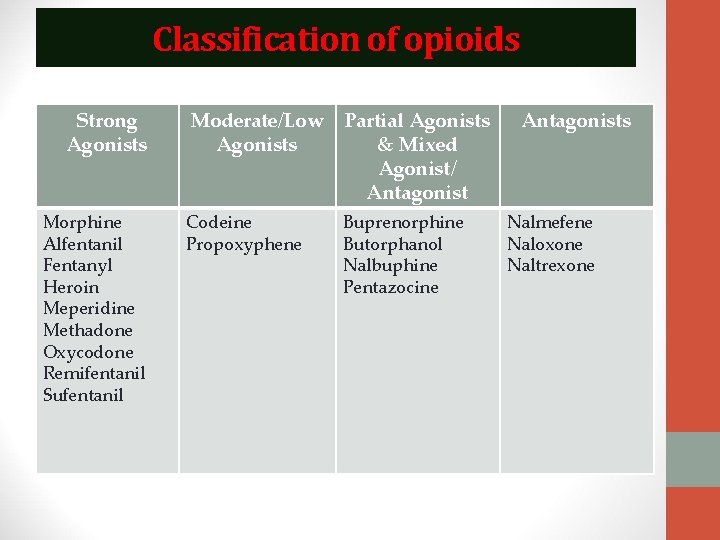
Classification of opioids Strong Agonists Morphine Alfentanil Fentanyl Heroin Meperidine Methadone Oxycodone Remifentanil Sufentanil Moderate/Low Agonists Partial Agonists & Mixed Agonist/ Antagonist Codeine Propoxyphene Buprenorphine Butorphanol Nalbuphine Pentazocine Antagonists Nalmefene Naloxone Naltrexone

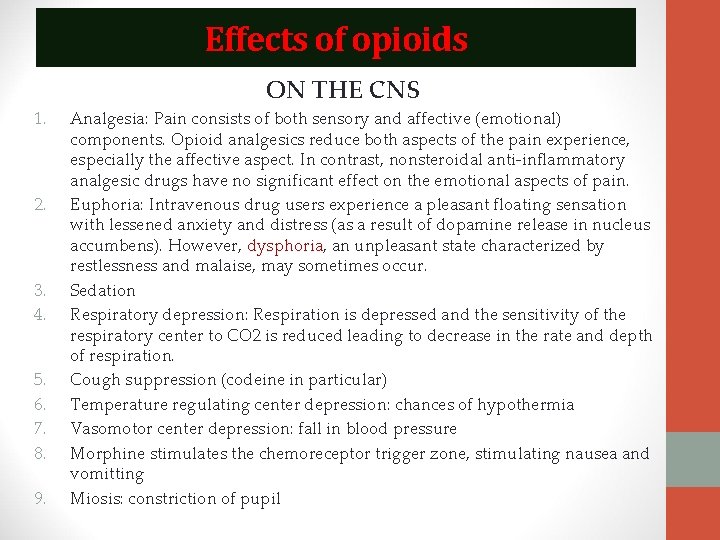
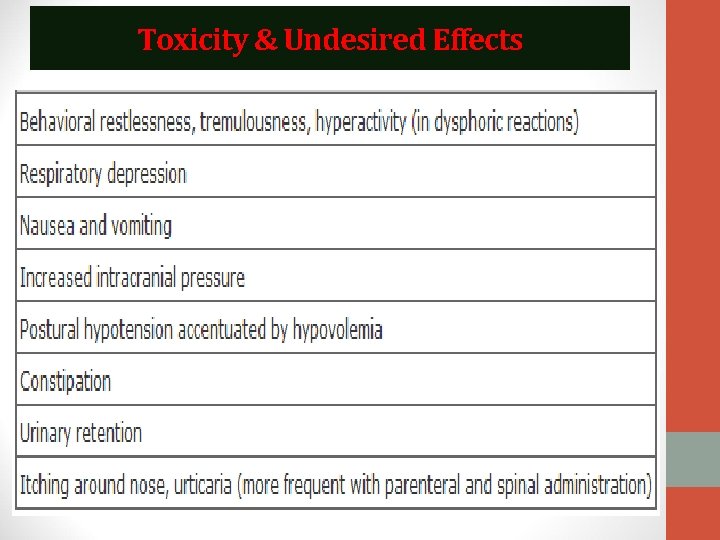
Effects of opioids ON THE CNS 1. 2. 3. 4. 5. 6. 7. 8. 9. Analgesia: Pain consists of both sensory and affective (emotional) components. Opioid analgesics reduce both aspects of the pain experience, especially the affective aspect. In contrast, nonsteroidal anti-inflammatory analgesic drugs have no significant effect on the emotional aspects of pain. Euphoria: Intravenous drug users experience a pleasant floating sensation with lessened anxiety and distress (as a result of dopamine release in nucleus accumbens). However, dysphoria, an unpleasant state characterized by restlessness and malaise, may sometimes occur. Sedation Respiratory depression: Respiration is depressed and the sensitivity of the respiratory center to CO 2 is reduced leading to decrease in the rate and depth of respiration. Cough suppression (codeine in particular) Temperature regulating center depression: chances of hypothermia Vasomotor center depression: fall in blood pressure Morphine stimulates the chemoreceptor trigger zone, stimulating nausea and vomitting Miosis: constriction of pupil

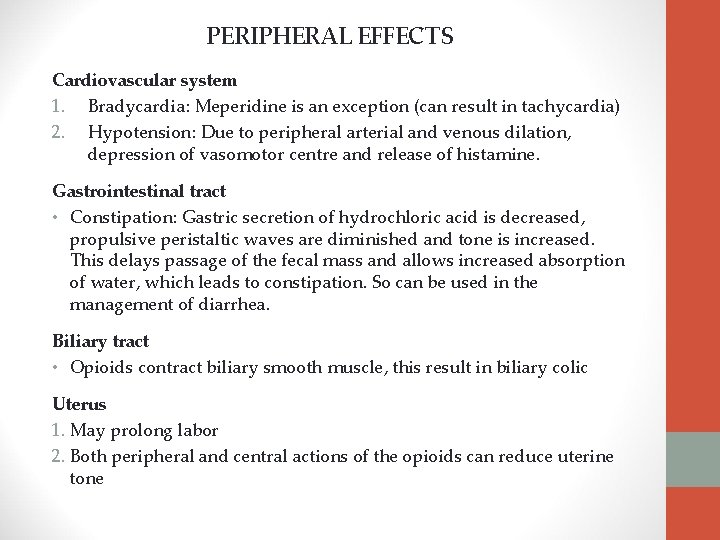
PERIPHERAL EFFECTS Cardiovascular system 1. Bradycardia: Meperidine is an exception (can result in tachycardia) 2. Hypotension: Due to peripheral arterial and venous dilation, depression of vasomotor centre and release of histamine. Gastrointestinal tract • Constipation: Gastric secretion of hydrochloric acid is decreased, propulsive peristaltic waves are diminished and tone is increased. This delays passage of the fecal mass and allows increased absorption of water, which leads to constipation. So can be used in the management of diarrhea. Biliary tract • Opioids contract biliary smooth muscle, this result in biliary colic Uterus 1. May prolong labor 2. Both peripheral and central actions of the opioids can reduce uterine tone

Renal • Renal function is depressed by opioids • Decreased renal plasma flow • Enhanced renal tubular sodium reabsorption • Ureteral and bladder tone are increased • Increased sphincter tone may precipitate urinary retention • Ureteral colic caused by a renal calculus is made worse by opioid-induced increase in ureteral tone Neuroendocrine • Stimulate the release of ADH, prolactin, and somatotropin • Inhibit the release of luteinizing hormone Pruritus • CNS effects and peripheral histamine release may be responsible for these reactions • Pruritus and occasionally urticaria (when administered parenterally) Miscellaneous The opioids modulate the immune system by • Lymphocyte proliferation • Antibody production • Chemotaxis

Clinical use and contraindications • • Analgesia Cough Diarrhea Acute Pulmonary Edema Balanced anaesthesia Preanaesthetic medication Relief of anxiety and apprehension Contraindications • Use in Patients with head injuries: Causes marked respiratory depression. Carbon dioxide retention caused by respiratory depression results in cerebral vasodilation. • In pregnancy: In pregnant women who are chronically using opioids, the fetus may become physically dependent in utero and manifest withdrawal symptoms in the early postpartum period. • Patients with impaired hepatic or renal function • Patients with endocrine disease • In bronchial asthma due to bronchoconstriction, release of histamine and respiratory depression •

Toxicity & Undesired Effects

Acute morphine poisoning • Its caused by a dose of >50 mg of morphine • Lethal dose is 250 mg • Clinical features include: Stupor , Coma , Shallow breathing , Cyanosis and sweating, Pinpoint pupil , Fall in BP, Convulsions, Death due to respiratory failure Treatment 1. Gastric lavage with potassium permanganate 0. 2% to oxidize the alkaloid (even if morphine was given by injection) + saline purgative, Mg SO 4 to evacuate the intestine. 2. Artificial respiration with O 2 – CO 2 mixture. 3. Mild respiratory stimulant may be used 4. i. v fluids 5. Specific antidotes: naloxone and naltrexone (opioid antagonists)

Tolerance and Dependence • With frequently repeated therapeutic doses of morphine, there is a gradual loss in effectiveness • To reproduce the original response, a larger dose must be administered (Tolerance). Along with tolerance, physical dependence develops • Physical dependence is defined as a characteristic withdrawal or abstinence syndrome when a drug is stopped or an antagonist is administered • The withdrawal symptoms are characterized by: • Yawning. • Rhinorrhea (watery discharge from the nose). • Lacrimation. • Restless sleep and insomnia. • Dilated pupils. • Vomiting. • Diarrhea. • Severe muscle cramps and headaches • Excitement. • Patient refuses food leading to dehydration and acidosis.

Causes of withdrawal symptoms • Enkephalins and endorphins are endogenous morphine-like compounds in the body. Enkephalins are present in the brain and GIT while endorphins are present in the pituitary. • If morphine is given externally, the opiate receptors become saturated (or overloaded) leading to inhibition of the synthesis of the endogenous morphine-like compounds. • If the exogenous opiate administration is stopped, withdrawal symptoms will occur, because the endogenous opioid is deficient and the receptor is deprived from both the endogenous and the exogenous opioids.

Treatment of morphine addiction 1. Hospitalization and psychotherapy 2. Gradual withdrawal of morphine till a stabilizing dose is attained which is just sufficient to prevent withdrawal symptoms from occurring. 3. Substitution therapy with methadone: • In the ratio of 1 mg methadone for 4 mg morphine for 1 week. • Then, methadone is withdrawn gradually over a period of 3 days. • It is an addictive drug but the withdrawal symptoms of methadone are less than those of morphine. 2. Hypnotics to help sleeping.

Opioid antagonists Naloxone: It is a competitive antagonist at all opioid receptors. It is a pure narcotic antagonist at all opioid receptor sites with no morphine like properties i. e. it is a pure antagonist. It has a rapid onset (1 -5 minutes) and a short duration of action. It is given only by injection either intravenously or subcutaneously. Naltrexone: has a longer duration of action than naloxone and a single oral dose of naltrexone blocks the effects of injected heroin up to 24 hours. (It is given orally) Therapeutic Uses: 1. Treatment of acute morphine poisoning (it stimulates respiration, improves miosis, vomiting and G. I spasm). 2. Diagnosis of opium addiction (precipitate withdrawal symptoms). Subcutaneous injection of 3 mg naloxone to an addict will precipitate withdrawal symptoms within ½ an hour. 3. Decreases the neonatal respiratory depression secondary to administration of morphine to the mother, because it can traverse the placental barrier. It is given to the mother before delivery or to the infant through the umbilical vein after delivery.

Analgesic Antipyretics (NSAIDs) • Non-steroidal anti-inflammatory drugs (NSAIDs) are a group of drugs that share in common the capacity to induce: 1. Analgesic effect. 2. Antipyretic effect. 3. Anti-inflammatory effect. • This group possess a common mode of action which is to block prostaglandin biosynthesis by inhibiting cyclo-oxygenase enzyme. • Inhibition of this enzyme centrally produce the analgesic antipyretic effect of this group. • While inhibition of this enzyme peripherally produce its anti inflammatory effect. • Paracetamol inhibit this enzyme centrally only so it has no antiinflammatory effect. It has only analgesic and antipyretic effects. It is not included in NSAIDs

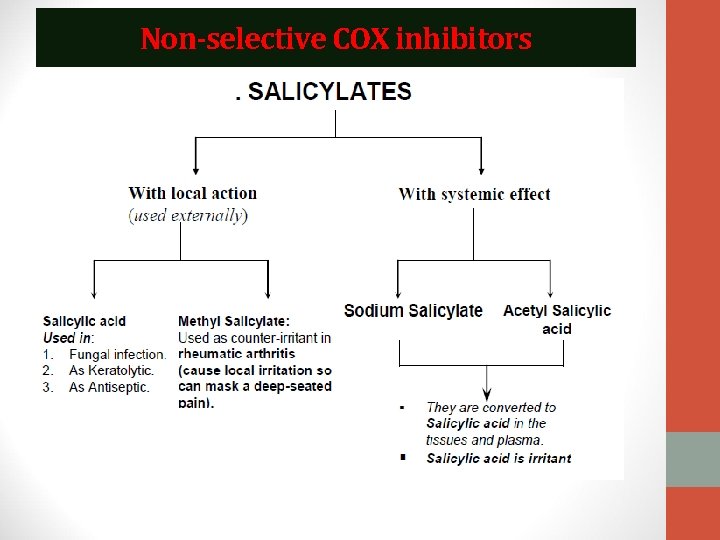
Classification of NSAIDs I. Non-selective COX inhibitors: These NSAIDs inhibit the constitutive COX-1 and the inducible COX-2 so are liable to be associated with gastrointestinal tract upset and renal impairment on long term use. • This group is further classified according to chemical structure into: i. Salicylates e. g. acetyl salicylic acid. ii. Other NSAIDs: a) Ibuprofen. b) Piroxicam (Feldene) c) Diclofenac d) Indomethacin. II. Selective COX-2 inhibitors: These NSAIDs selectively inhibit COX-2 and are less liable to be associated with side effects. Example: Celecoxib: • Celecoxib is a selective COX-2 inhibitor that spares COX-1, so it does not inhibit the synthesis of the protective prostaglandin in the gut. Hence, its anti-inflammatory effect is associated with less gastrointestinal adverse effects.

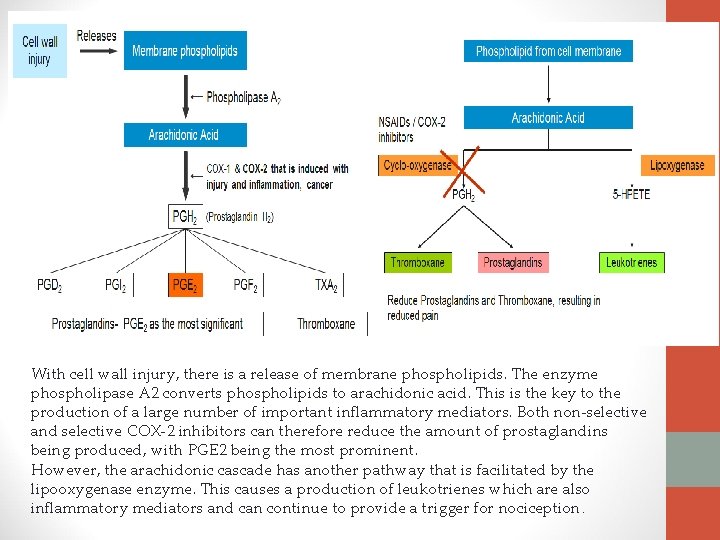
With cell wall injury, there is a release of membrane phospholipids. The enzyme phospholipase A 2 converts phospholipids to arachidonic acid. This is the key to the production of a large number of important inflammatory mediators. Both non-selective and selective COX-2 inhibitors can therefore reduce the amount of prostaglandins being produced, with PGE 2 being the most prominent. However, the arachidonic cascade has another pathway that is facilitated by the lipooxygenase enzyme. This causes a production of leukotrienes which are also inflammatory mediators and can continue to provide a trigger for nociception.

Non-selective COX inhibitors

Pharmacological actions of systemic salicylates 1. Analgesic action: Salicylates produce their analgesic action: a. By raising the threshold to painful stimuli relayed from the thalamus to the sensory cortex. b. Through its anti-inflammatory action because inflammed tissue will stimulate the pain receptors. 2. Antipyretic action: Acting on heat regulating center in the hypothalamus, salicylates promote heat loss by: a. Vasodilatation of cutaneous blood vessels stimulating radiation. b. Increasing sweating and encouraging evaporation. c. Mobilization of fluids from tissues to blood. a. Anti-inflammatory and anti-rheumatic action : Salicylates relieves muscular pain, joint pain and swelling. This effect is due to decreasing the synthesis of prostaglandins b. On the G. I. T: Salicylates irritate gastric mucosa leading to nausea and vomiting. Higher doses may cause gastric ulceration, and bleeding.

5. Uricosuric action: Small doses (less than 5 g /day) depress uric acid secretion by proximal tubules causing uric acid retention and large doses (greater than 5 g/day) inhibit the renal tubular reabsorption of uric acid by proximal tubules, increasing uric acid excretion, decreasing its plasma level thus producing uricosuric action. 6. Effect on the blood: • Decrease in sedimentation rate when elevated. • Decrease leucocytic count when elevated as in rheumatic fever. • Inhibits platelet aggregation secondary to inhibition of platelet COX and consequently reduces production of TXA 2 (a platelet aggregating factor) with consequent prolongation of bleeding time. • Large doses lead to hypoprothrombinaemia and prolongation of coagulation time. This effect is reversed by vitamin K.

Therapeutic uses 1. 2. 3. 4. 5. 6. 7. Fever (non-specific). Analgesic (headache, toothache, myalgia and arthralgia). Common cold (lowers fever and relieves headache and muscle aches). In gout Rheumatoid arthritis. Acute rheumatic fever (to relieve fever, arthritis but not the cardiac complications). Reducing the risk of myocardial infarction. Aspirin in low dose (75 - 150 mg/ day or lower) inhibits platelet aggregation i. e. anti-thrombotic.

Side effects and toxicity 1. Gastric irritation: Nausea, Vomiting, Epigastric discomfort. 2. Gastrointestinal bleeding and peptic ulceration. 3. Salicylism: (occurs after repeated administration of large doses of salicylates as in rheumatic fever and gout). Its symptoms are: Headache, Mental confusion, Ringing in the ears, Visual disturbances, Sweating, Nausea, vomiting and diarrhea. These effects disappear after stoppage of the drug. 4. Hypersensitivity: Aspirin allergy manifests itself in the form of: • Bronchoconstriction. • Angioneurotic edema (allergic edema). • Urticaria. Mechanism: by inhibiting COX enzyme, therefore arachidonic acid will be acted upon by lipooxygenase enzyme increasing leukotrienes (powerful bronchoconstrictor). • Aspirin is contraindicated in these hypersensitive patients.

5. Reye's syndrome: In which there is severe hepatic injury and encephalopathy may occur following the use of salicylates to control fever in viral infections in children. 6. Long term abuse of analgesic mixtures may lead to nephropathy. 7. Acute salicylate poisoning: Clinical features include (a) G. I. T: Nausea, Vomiting. (b) C. N. S: Restlessness, Tremors, Convulsions, Coma. (c) Hemorrhages. (d) Hyperpyrexia. (e) Hyperglycemia. (f) Acid/ base imbalance: Respiratory alkalosis Treatment of acute salicylate Poisoning: 1. Gastric lavage. 2. Correction of hyperthermia by cold water fomentation. 3. Correction of dehydration by I. V. fluids. 4. For hemorrhage: Vitamin K or blood transfusion. 5. Alkalinization of urine by intravenous injection of Na. HCO 3 to increase the excretion of salicylates. 6. Hemodialysis in severe cases.

Contraindications 1. 2. 3. 4. 5. 6. In peptic ulcer. Patients having hypersensitivity reactions to salicylates. Bleeding tendency. Patients taking anti-coagulants. Children with viral infections e. g. chicken pox or influenza. Gout: in small dose.

Paracetamol • It is an analgesic-antipyretic with no anti-inflammatory action. It effectively inhibits PG synthesis in the CNS resulting in analgesic and antipyretic effects but it is a weak PG inhibitor in peripheral tissue thus has no anti-inflammatory effect. Actions: 1. Analgesic antipyretic (inhibits COX enzyme centrally). 2. No anti-inflammatory effect (does not inhibit this enzyme peripherally). 3. No uricosuric effect. Therapeutic Uses: • Paracetamol is a commonly used analgesic antipyretic instead of aspirin in cases of: • 1. Peptic or gastric ulcers (it causes no GIT disturbances) • 2. Bleeding tendency, (it does not affect platelet function) • 3. Allergy to salicylates. • 4. Viral infections in children (to avoid Reye syndrome). Toxicity: • 1. Skin rash. • 2. Liver damage (with over dosage).