Paediatric ITP Philip Connor Brief Background autoimmune disorder

Paediatric ITP Philip Connor

Brief Background • • autoimmune disorder of platelet destruction, mucocutaneous bleeding episodes with a platelet count <100 x 109/L. majority of paediatric patients will spontaneously recover with conservative management. minority require treatment for bleeding, usually occurring at a platelet count of <20 x 109/L. • • Peaks of new cases in Winter months – driven by viral URTIs Persistence/Chronicity in 30% children vs. 70% of adults • Similar incidence as Paediatric ALL - 2 -3/100000 person years – about 400/year in UK • Persistent if > 3 months, Chronic if >12 months • ITP registries in the UK for both Adult and Paediatric age groups – • Both are NIHR portfolio adopted studies Other international registries exist – International Cooperative Study Group (ICIS) the biggest – 131 centres in multiple countries

The natural course of Childhood immune thrombocytopenia: results from the paediatric ITP registry Dr John Grainger, Eilish Hannah

About This Study • This is the second large prospective study to follow children with ITP for longer than 12 months • It is the first national prospective study to follow children with ITP for longer than 12 months • Intercontinental cooperative ITP study group (ICIS produced the first) • This study adds further knowledge to the natural course of the disease that was not reflected in the ICIS study. • The UK registry captures patients of all severities whereas the ICIS cohort may reflect patients with more severe ITP. • this study is more likely to have uniform management for each patient

Objectives To map the natural course of paediatric ITP with regards to: • Recovery • Platelet counts at specific time intervals • Bleeding risk and bleeding severity

Methods • This study used data collected from the UK paediatric ITP registry between January 2007 and February 2013. • The registry has input from 80 different sites across the UK • 564 patients were included aged 2 months to 16 years • Mild, moderate, severe and life-threatening bleeding severity was defined by the Bolton-Maggs bleeding score. • This study defined recovered as three consecutive platelet readings over 150 x 109/L. If a patient had a reading over 150 x 109/L and then a further reading below 150 x 109/L they were not classed as recovered.

Key Differences between this Study and the ICIS study. • We had a smaller percentage of patients recovering at each time interval. • Our study found patients had much lower platelet counts in the initial 6 months, 12 months and 24 months. • This is mainly explained by the differences in management between the two cohort of patients.
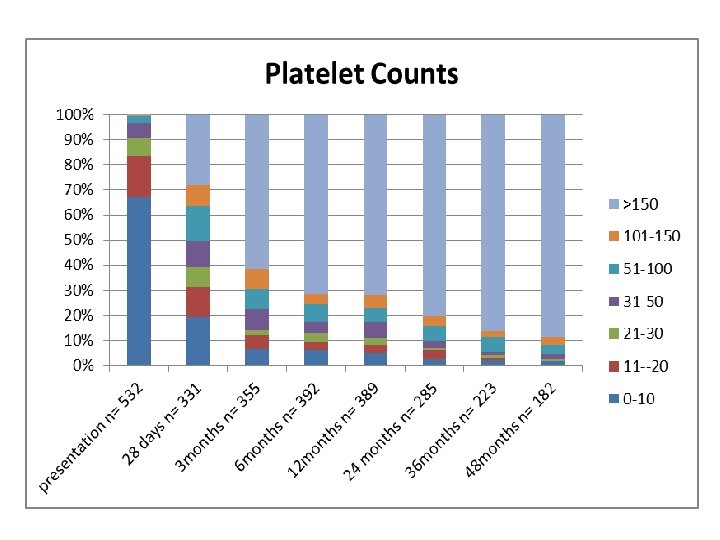
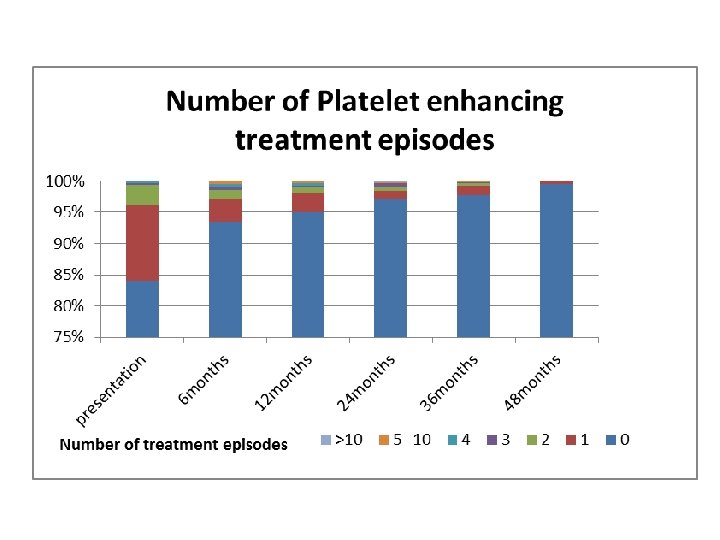
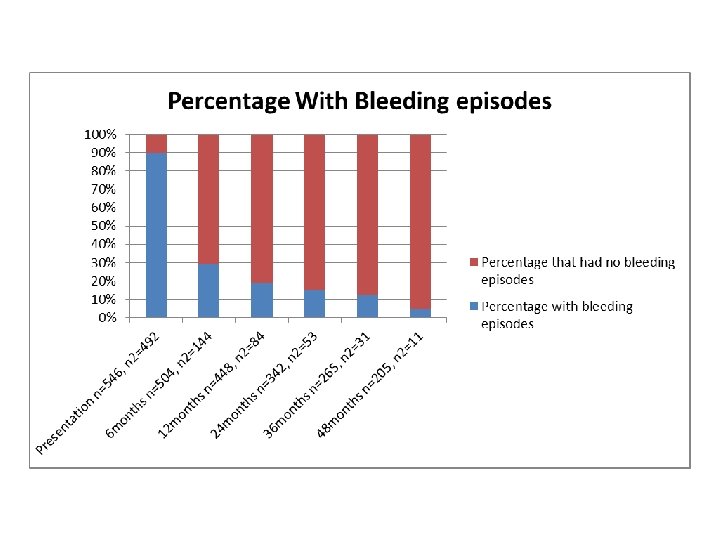

Ways Our Study Supported the ICIS study • Platelet counts despite the differences mentioned earlier did follow a similar pattern, of an inverse relationship; as time progressed the percentage of patients with a low platelet count decreased. • Like the ICIS study we also found the most common bleeding site to be the skin • The percentage that developed chronic ITP was similar.
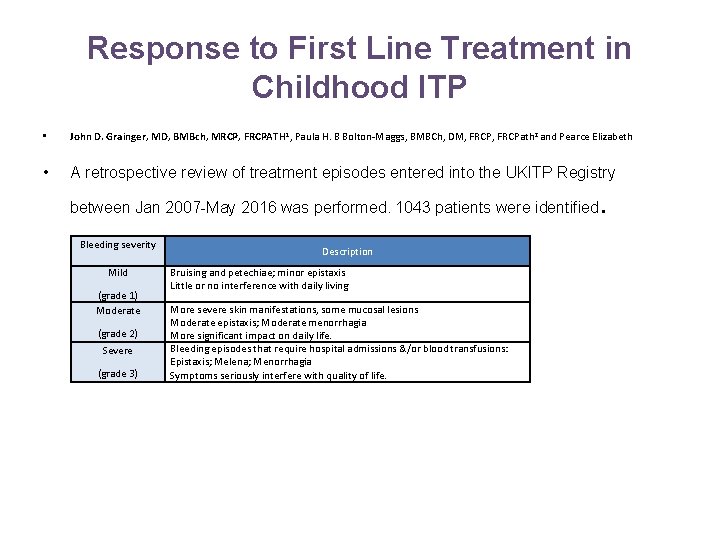
Response to First Line Treatment in Childhood ITP • John D. Grainger, MD, BMBch, MRCP, FRCPATH 1, Paula H. B Bolton-Maggs, BMBCh, DM, FRCPath 2 and Pearce Elizabeth • A retrospective review of treatment episodes entered into the UKITP Registry between Jan 2007 -May 2016 was performed. 1043 patients were identified Bleeding severity Mild (grade 1) Moderate (grade 2) Severe (grade 3) Description Bruising and petechiae; minor epistaxis Little or no interference with daily living More severe skin manifestations, some mucosal lesions Moderate epistaxis; Moderate menorrhagia More significant impact on daily life. Bleeding episodes that require hospital admissions &/or blood transfusions: Epistaxis; Melena; Menorrhagia Symptoms seriously interfere with quality of life. .

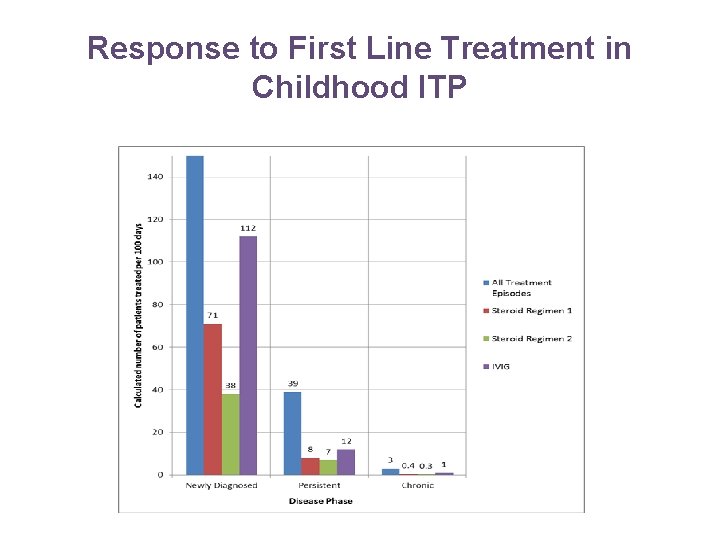
Response to First Line Treatment in Childhood ITP • 798 patients were untreated other than supportive care. • 245 patients (23%) had 422 treatment episodes for bleeding episodes. Of these 351 were single therapies and 71 were combination therapies. Treatment • 136 treatments were with IVIG 152 treatments were Prednisolone • • 92 Regimen 1: 1 -2 mg/kg/day for 7 -14 days 60 Regimen 2: 4 mg/kg/day for 4 days. 71 treatments were with combination therapy
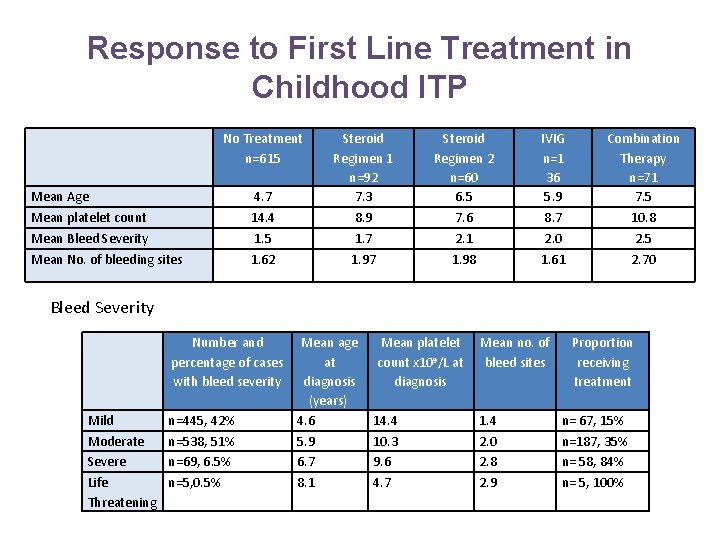
Response to First Line Treatment in Childhood ITP No Treatment n=615 Mean Age Mean platelet count Mean Bleed Severity Mean No. of bleeding sites 4. 7 14. 4 1. 5 1. 62 Steroid Regimen 1 n=92 7. 3 8. 9 1. 7 1. 97 Steroid Regimen 2 n=60 6. 5 7. 6 2. 1 1. 98 IVIG n=1 36 5. 9 8. 7 2. 0 1. 61 Combination Therapy n=71 7. 5 10. 8 2. 5 2. 70 Bleed Severity Number and percentage of cases with bleed severity Mild n=445, 42% Mean age at diagnosis (years) 4. 6 Moderate Severe n=538, 51% n=69, 6. 5% 5. 9 6. 7 10. 3 9. 6 2. 0 2. 8 n=187, 35% n= 58, 84% 8. 1 4. 7 2. 9 n= 5, 100% Life n=5, 0. 5% Threatening Mean platelet count x 109/L at diagnosis Mean no. of bleed sites Proportion receiving treatment 14. 4 1. 4 n= 67, 15%
Response to First Line Treatment in Childhood ITP
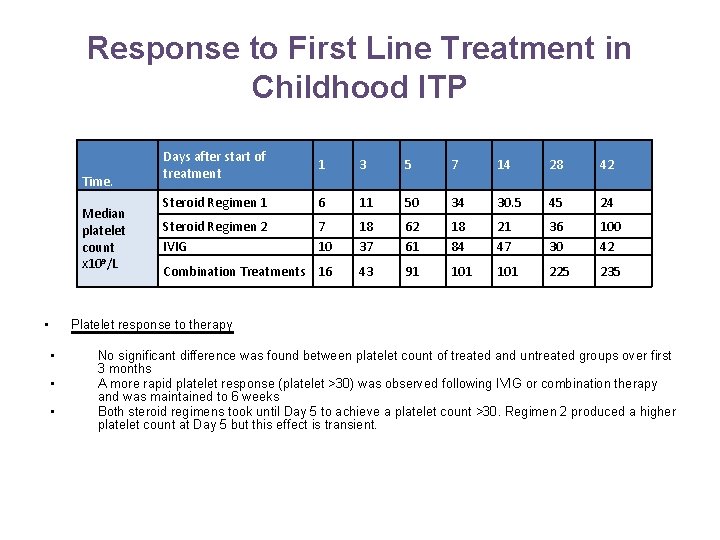
Response to First Line Treatment in Childhood ITP Time. Median platelet count x 109/L • Days after start of treatment 1 3 5 7 14 28 42 Steroid Regimen 1 6 11 50 34 30. 5 45 24 Steroid Regimen 2 IVIG 7 10 18 37 62 61 18 84 21 47 36 30 100 42 Combination Treatments 16 43 91 101 225 235 Platelet response to therapy • • • No significant difference was found between platelet count of treated and untreated groups over first 3 months A more rapid platelet response (platelet >30) was observed following IVIG or combination therapy and was maintained to 6 weeks Both steroid regimens took until Day 5 to achieve a platelet count >30. Regimen 2 produced a higher platelet count at Day 5 but this effect is transient.

Response to First Line Treatment in Childhood ITP • • In the UK, 23% of children with ITP are receiving treatment. A higher proportion required treatment with increasing bleeding severity and number of bleeding sites. • IVIG demonstrated a superior response to steroid therapy and should be considered preferable for more significant bleeding. • A higher dose, short duration steroid regimen was associated with a slightly faster response compared to lower dose treatment and may be preferable to longer duration steroids for milder bleeding that requires therapy.

Change Natural History • Our registry data suggests no change • ‘Treatment with or without IVIg for Kids with ITP’ (TIKI) trial (NTR study ID TC 1563) – Between May 2009 and May 2015, 200 patients were enrolled, 109 males and 91 females. After randomization, 100 received IVIg and 100 received careful observation – No statistically significant differences were seen regarding development of chronic ITP (currently defined as a platelet count <100 x 109/L at 12 months) between both groups: 10. 2% in the IVIg group and 10. 4% in the observation group

New Agents • TPO mimetics used in c. ITP in children. – Eltrombopag – • efficacy shown in the PETIT Studies • Licensed in USA and Europe – Romiplstim • Current study underway “A Single-Arm, Open-Label, Long-Term Efficacy and Safety Study of Subcutaneous (SC) Romiplostim in Children with Immune Thrombocytopenia (ITP)”
New Agents • Empiric observation that the TPO mimetics seem to change the natural history of Chronic ITP • 30% remission rate per year (vs. 10% expected) • ? Upfront use in acute ITP?

Acknowledgments 1. Slides from John Grainger 2. ITP Support Association for their on going funding of the ITP registry. 3. The UK Paediatric ITP Registry is currently supported by 80 treatment centres across the UK. Details of all centres are available at www. uk-itp. org. 4. Medicines for children research network 5. University of Manchester
- Slides: 21