PACKET 1 Math Lab Skills AtlanticPacific Rule Density
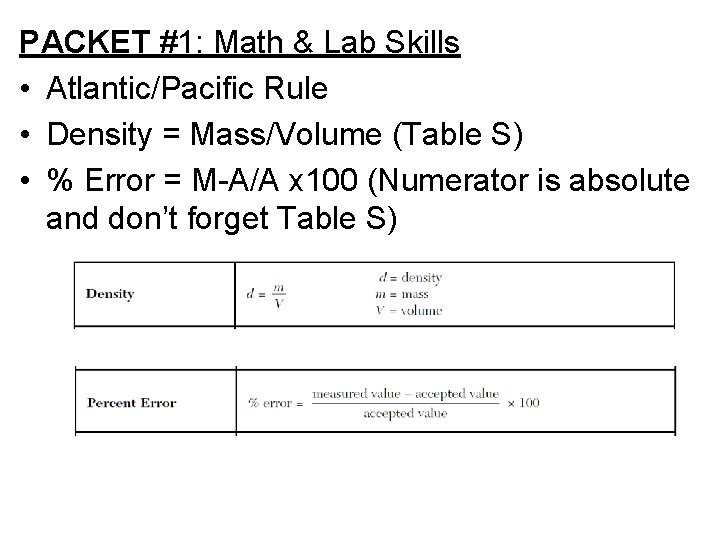
PACKET #1: Math & Lab Skills • Atlantic/Pacific Rule • Density = Mass/Volume (Table S) • % Error = M-A/A x 100 (Numerator is absolute and don’t forget Table S)

PACKET #2: Energy & Thermodynamics • • • Solid (s) – Crystalline Aqueous (aq) – Homogenous Mixture S L G = Endothermic G L S = Exothermic Diatomic Molecules – MUST KNOW THEM! • Compound – can be broken down (chemically combined/fixed) • Element – can’t be broken down • Mixture – Physically combined/varied
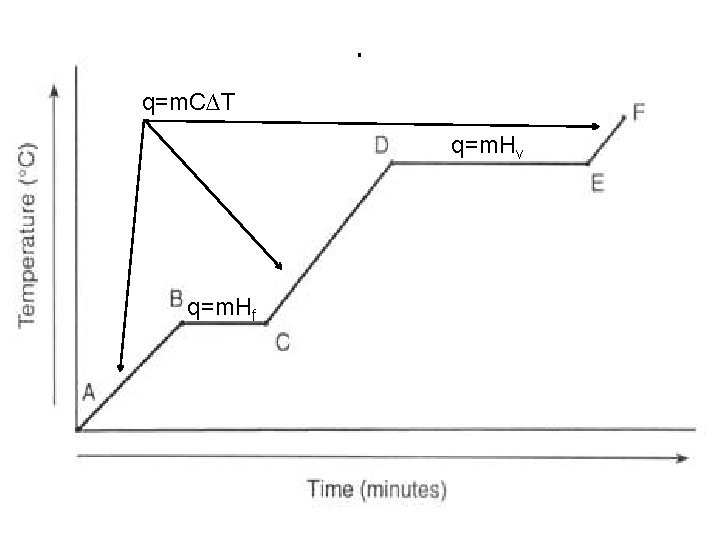
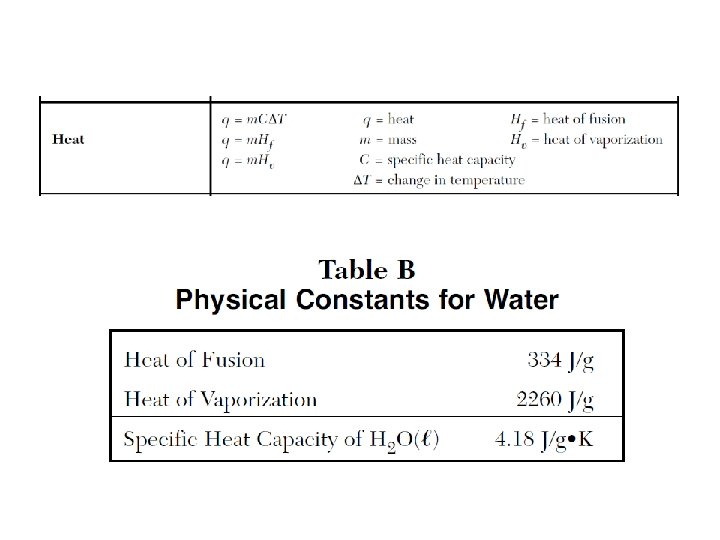
q=m. C∆T I q=m. Hv q=m. Hf
PACKET #3: Atomic Concepts • • Proton - +1, 1 amu, Nucleus (Atomic #) Neutron – 0, 1 amu, Nucleus (At. #-Mass#) Electron - -1, 0 amu, Orbital Atomic # - Protons Mass # - Proton + Neutron Nuclear Charge - #Protons Isotope – Same # protons, Different # neutrons Atomic Mass – Average of all the natually occurring isotopes. • Ion – Charged Particles (+) lose, (-) gain. Why solutions are good conductors of electricity.
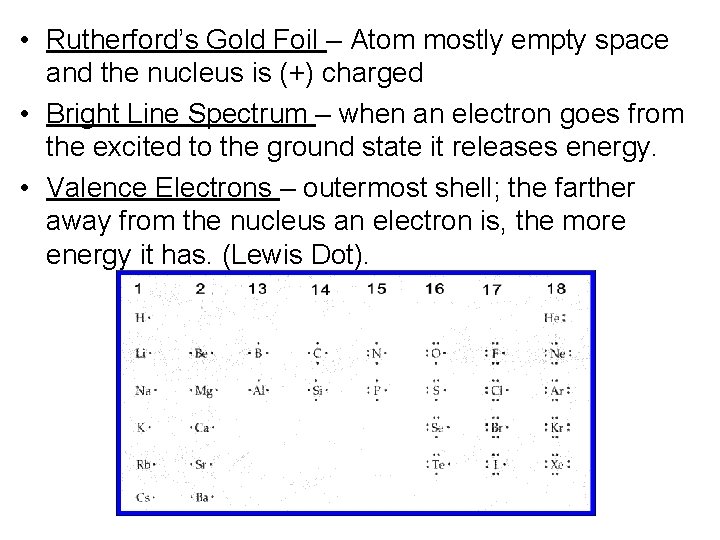
• Rutherford’s Gold Foil – Atom mostly empty space and the nucleus is (+) charged • Bright Line Spectrum – when an electron goes from the excited to the ground state it releases energy. • Valence Electrons – outermost shell; the farther away from the nucleus an electron is, the more energy it has. (Lewis Dot).

PACKET #4: Periodic Table • Arranged in increasing Atomic #. • Groups – vertical, same # valence electrons (more similar) • Periods – horizontal, same # PEL’s. • Metals – left of zig zag (metallic bonds, mobile electrons) • Non-metals – rights of zig zag • Metalloids – on zig zag • Noble Gases – Group 18 (stable valence shell) • Liquids – Hg & Br • Gases – Noble gases and H 2, N 2, O 2, F 2, Cl 2. • Allotropes – Oxygen (O 2, O 3) and Carbon (coal, diamond, graphite). Same element with different

TABLE S: Please remember that you can always refer to Table S to determine this!! • Electronegativity – attraction for electrons (decreases down a group, increases across a period) • Ionization Energy – energy needed to lose electrons (decreases down a groups, increases across a period) • Atomic Radius – distance from nucleus to valence shell (increases down a group, decreases across a period)
PACKET #5: Naming/Balancing/Reaction Types • Ionic – Ions transfer electrons – M-NM, M-PI, PI-NM, PI-PI (if there’s a PI then it’s BOTH) – Criss-Cross Method – Roman Numerals for metals with multiple Ox. #. Roman numerals indicate the Ox. # • Covalent – NM’s electrons are transferred

• Chemical Reactions have conservation of Mass, Charge, and Energy!! • BARF • Synthesis • Decomposition • Single Replacement (Table J) • Double Replacement • Combustion (Table I)
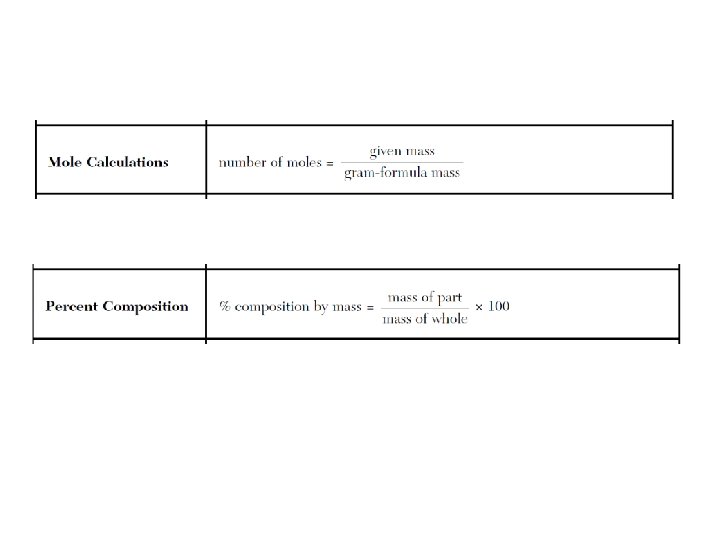
PACKET #6: Moles & Stiochiometry GFM = 1 mole = 6. 02 x 1023 molecules = 22. 4 L • • • Molecular Formula (Covalent) – Ex. C 6 H 12 O 6 Empirical Formula – Ex. CH 2 O (simplified) Mass MF/Mass EF Moles = given mass/GFM % composition = part/whole x 100 Hydrate • % composition = hydrate-anhydrate/hydrate x 100 • Stoichiometry – remember that the coefficients are the # of moles, and the ratio of the substances have
PACKET #7: Chemical Bonding • Ionic – EN difference greater than 1. 7, transfer electrons; ALWAYS POLAR (unequal electron distribution). Only good conductors of electricity as a liquid (l) or aqueous (aq). • Covalent – EN difference less than 1. 7, sharing electrons; Polar Bond (diff. EN); Non-Polar Bond (same EN – diatomic molecules) • SNAP (symmetrical non-polar; asymmetrical polar) – Polar (H 2 O, NH 3, HCl) vs. Non-Polar Molecule (CH 4, CO 2, Diatomics) • Partially + & - (Only in Asymmetrical Polar Molecules) – (-) has the higher EN, (+) has the lower EN • Network Solids – Diamond, Si. C, Si. O 2. • Metallic Bonds – Why metals are excellent conductors of electricity in the SOLID phase. Mobile electrons!!!
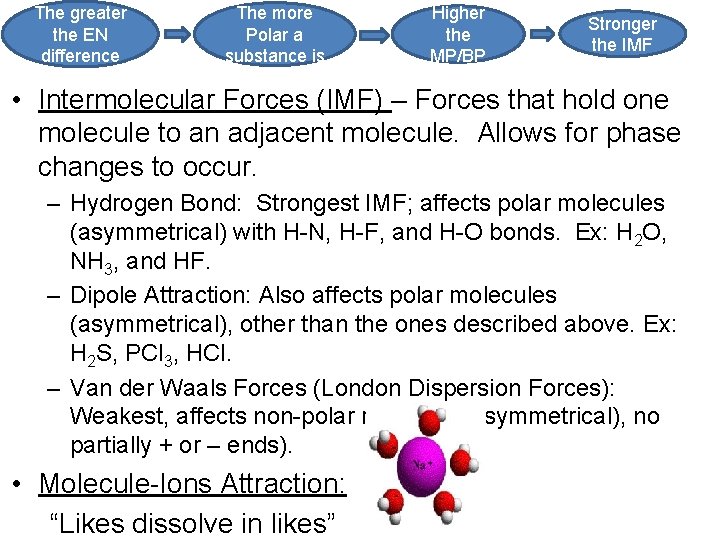
The greater the EN difference The more Polar a substance is Higher the MP/BP Stronger the IMF • Intermolecular Forces (IMF) – Forces that hold one molecule to an adjacent molecule. Allows for phase changes to occur. – Hydrogen Bond: Strongest IMF; affects polar molecules (asymmetrical) with H-N, H-F, and H-O bonds. Ex: H 2 O, NH 3, and HF. – Dipole Attraction: Also affects polar molecules (asymmetrical), other than the ones described above. Ex: H 2 S, PCl 3, HCl. – Van der Waals Forces (London Dispersion Forces): Weakest, affects non-polar molecules (symmetrical), no partially + or – ends). • Molecule-Ions Attraction: “Likes dissolve in likes”
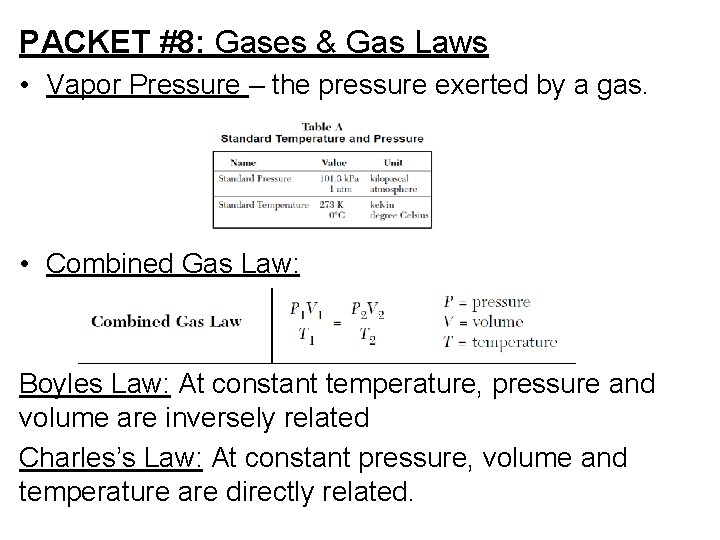
PACKET #8: Gases & Gas Laws • Vapor Pressure – the pressure exerted by a gas. • Combined Gas Law: Boyles Law: At constant temperature, pressure and volume are inversely related Charles’s Law: At constant pressure, volume and temperature are directly related.
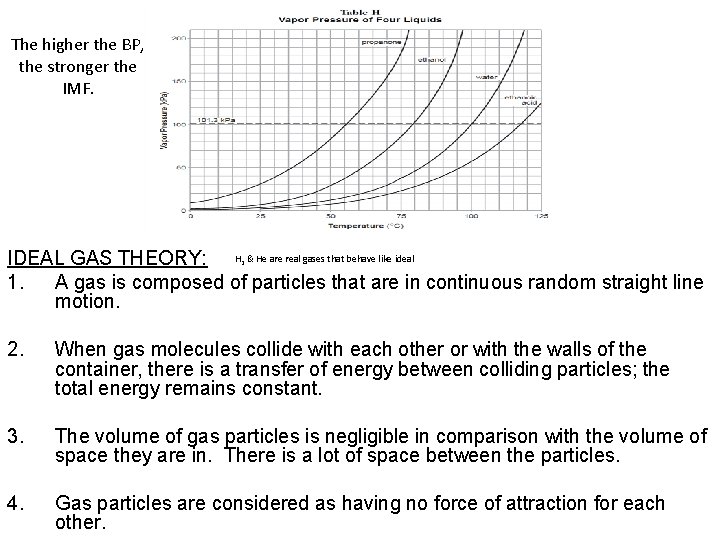
The higher the BP, the stronger the IMF. IDEAL GAS THEORY: H & He are real gases that behave like ideal 1. A gas is composed of particles that are in continuous random straight line motion. 2 2. When gas molecules collide with each other or with the walls of the container, there is a transfer of energy between colliding particles; the total energy remains constant. 3. The volume of gas particles is negligible in comparison with the volume of space they are in. There is a lot of space between the particles. 4. Gas particles are considered as having no force of attraction for each other.
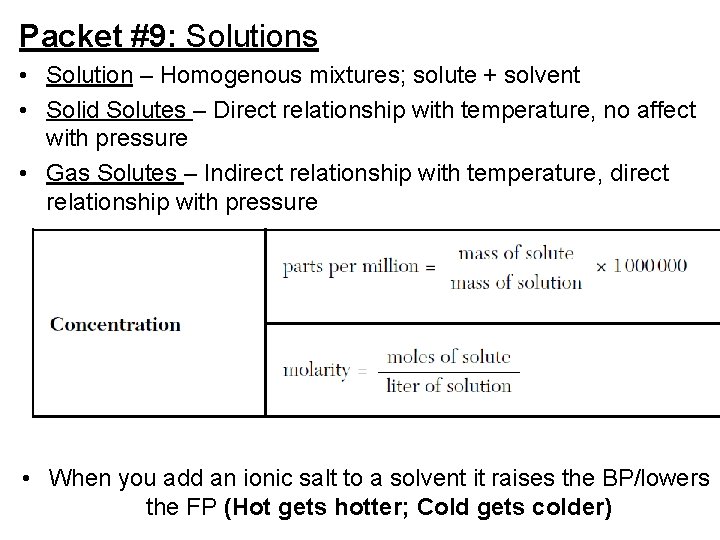
Packet #9: Solutions • Solution – Homogenous mixtures; solute + solvent • Solid Solutes – Direct relationship with temperature, no affect with pressure • Gas Solutes – Indirect relationship with temperature, direct relationship with pressure • When you add an ionic salt to a solvent it raises the BP/lowers the FP (Hot gets hotter; Cold gets colder)
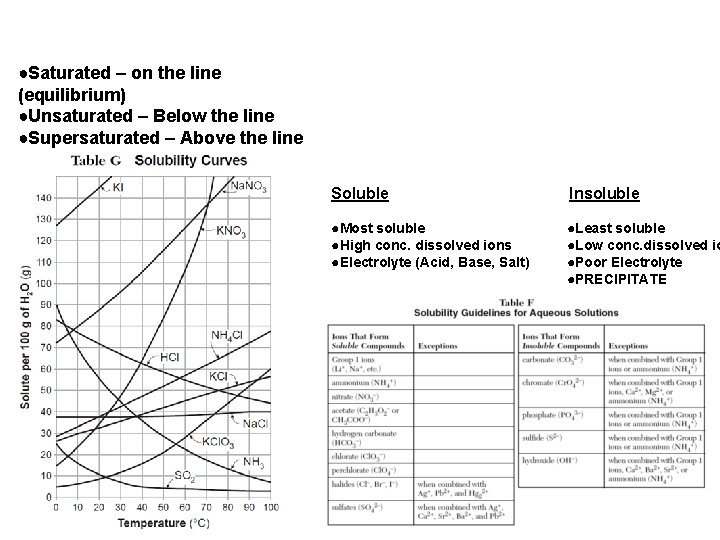
●Saturated – on the line (equilibrium) ●Unsaturated – Below the line ●Supersaturated – Above the line Soluble Insoluble ●Most soluble ●High conc. dissolved ions ●Electrolyte (Acid, Base, Salt) ●Least soluble ●Low conc. dissolved io ●Poor Electrolyte ●PRECIPITATE
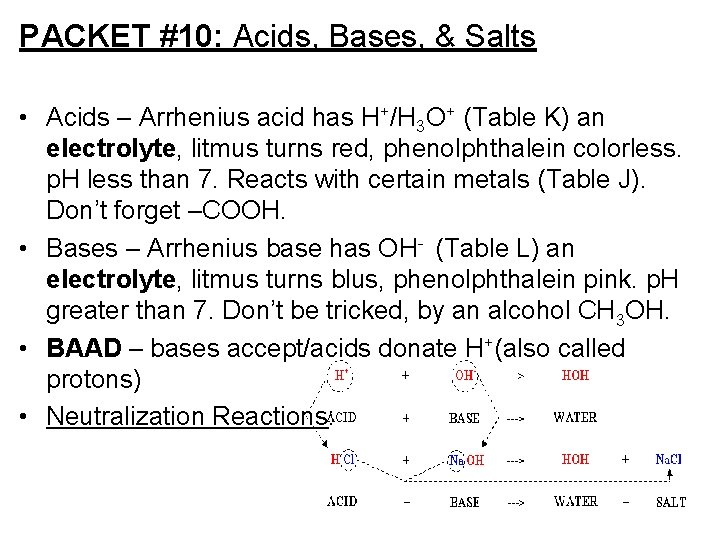
PACKET #10: Acids, Bases, & Salts • Acids – Arrhenius acid has H+/H 3 O+ (Table K) an electrolyte, litmus turns red, phenolphthalein colorless. p. H less than 7. Reacts with certain metals (Table J). Don’t forget –COOH. • Bases – Arrhenius base has OH- (Table L) an electrolyte, litmus turns blus, phenolphthalein pink. p. H greater than 7. Don’t be tricked, by an alcohol CH 3 OH. • BAAD – bases accept/acids donate H+(also called protons) • Neutralization Reactions:
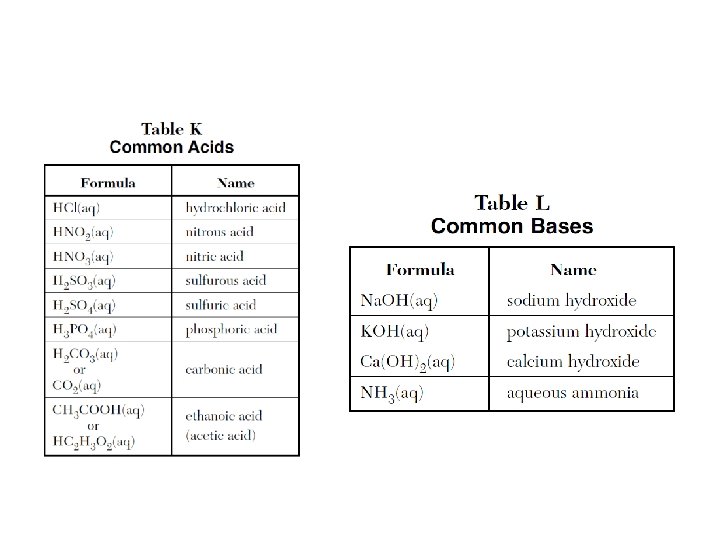
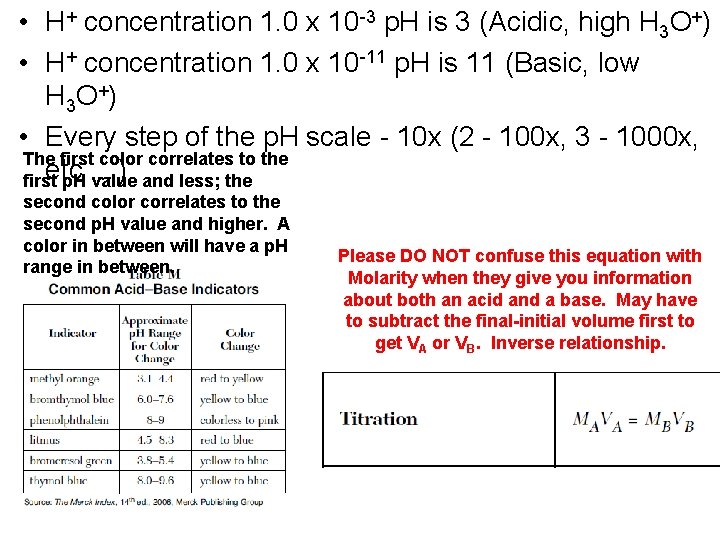
• H+ concentration 1. 0 x 10 -3 p. H is 3 (Acidic, high H 3 O+) • H+ concentration 1. 0 x 10 -11 p. H is 11 (Basic, low H 3 O + ) • Every step of the p. H scale - 10 x (2 - 100 x, 3 - 1000 x, The first color correlates to the etc first p. H …) value and less; the second color correlates to the second p. H value and higher. A color in between will have a p. H range in between. Please DO NOT confuse this equation with Molarity when they give you information about both an acid and a base. May have to subtract the final-initial volume first to get VA or VB. Inverse relationship.
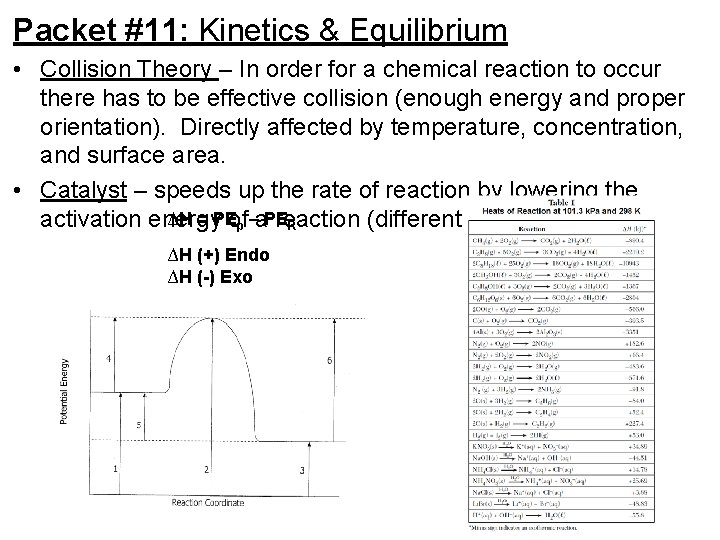
Packet #11: Kinetics & Equilibrium • Collision Theory – In order for a chemical reaction to occur there has to be effective collision (enough energy and proper orientation). Directly affected by temperature, concentration, and surface area. • Catalyst – speeds up the rate of reaction by lowering the ∆H = PEof activation energy reaction (different pathway). p –a. PE R ∆H (+) Endo ∆H (-) Exo

Equilibrium – the rate of the forward and the reverse reactions are equal; the concentrations (amounts) remain constant (phase, solution, chemical) – Phase: any phase change – Solution: saturated (dissolve = settle out) – Chemical: Le Chatelier’s Principle – when a chemical reaction at equilibrium is stressed (concentration, temperature, pressure) it shift away from that side in order to alleviate the stress and return to equilibrium. The side where more collisions occur is the side that’s stressed. – YOU MUST ALWAYS MENTION A SHIFT WHEN ASKED “EXPLAIN IN TERMS OF LE CHATELIER’S PRINCIPLE”!!!!! Spontaneous Reaction (occurs in nature): • Low Energy, High Entropy
PACKET #12: REDOX • A reaction in which the oxidation numbers change and the amount of electrons lost (oxidation) is EQUAL to the amount of electrons gained (reduction). “OIL RIG” • YOU MUST BE ABLE TO ASSIGN OXIDATION NUMBERS! • When an electron is lost, the electron is on the right side of the half reaction and the oxidation # goes UP. • When an electron is gained, the electron is on the left side of the half reaction and the oxidation # goes DOWN. • Single Replacement Reactions are ALWAYS REDOX. Double Replacement Reactions are NEVER REDOX. Remember, the element by itself has to be more reactive then the element its replacing. The more reactive metal is always the ANODE.
AN OX; RED CAT VOLTAIC ELECTROLYTIC • • Non-spontaneous • Electrical Chemical • Requires a battery as a source of energy • Anode – oxidation (+) • Cathode – reduction (-) • (+) charged ion moves toward the cathode • (-) charged ion moves toward the anode • Electroplating: the thing being plated is the cathode. Spontaneous Chemical Electrical Energy Anode – oxidation (-) Cathode – reduction (+) Example: BATTERY Electrons travel from anode to cathode (wire) • More reactive metal (Table J) ALWAYS the site of oxidation • Salt bridge is for the flow of ions from one half-cell to another
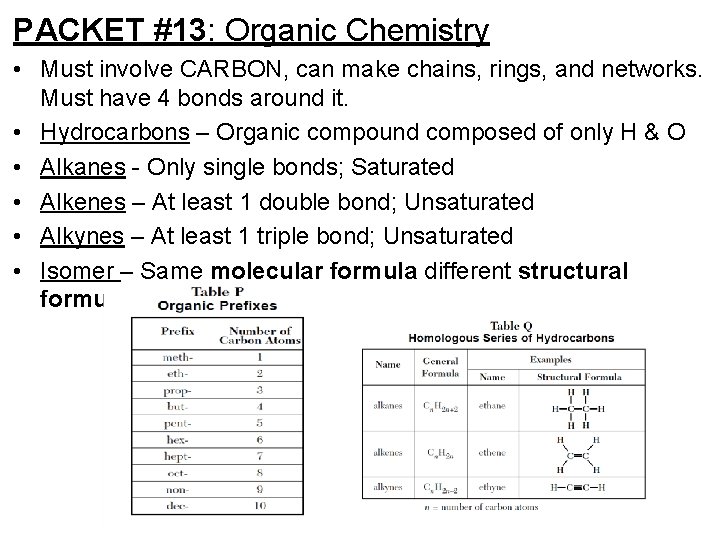
PACKET #13: Organic Chemistry • Must involve CARBON, can make chains, rings, and networks. Must have 4 bonds around it. • Hydrocarbons – Organic compound composed of only H & O • Alkanes - Only single bonds; Saturated • Alkenes – At least 1 double bond; Unsaturated • Alkynes – At least 1 triple bond; Unsaturated • Isomer – Same molecular formula different structural formula.
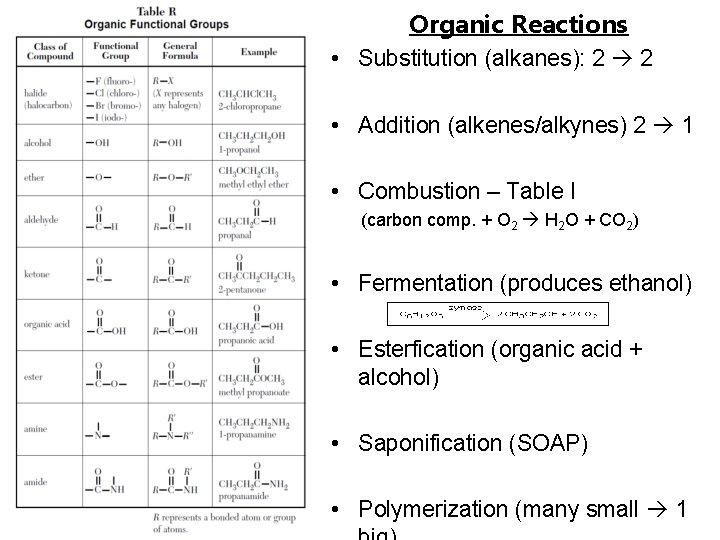
Organic Reactions • Substitution (alkanes): 2 2 • Addition (alkenes/alkynes) 2 1 • Combustion – Table I (carbon comp. + O 2 H 2 O + CO 2) • Fermentation (produces ethanol) • Esterfication (organic acid + alcohol) • Saponification (SOAP) • Polymerization (many small 1
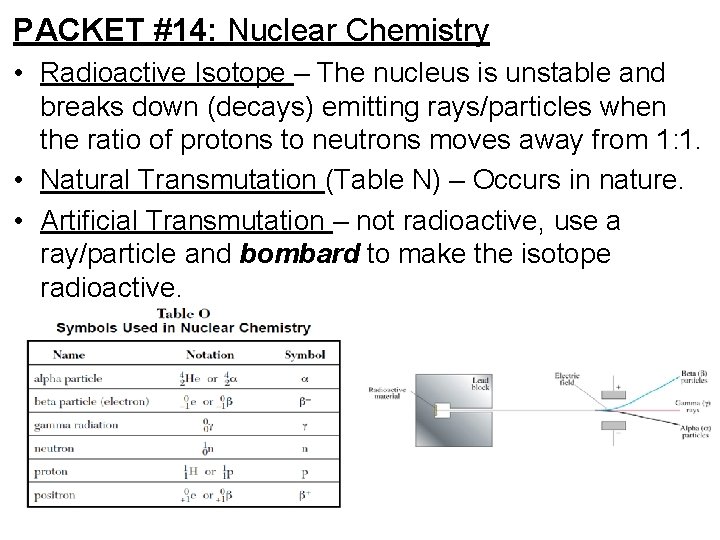
PACKET #14: Nuclear Chemistry • Radioactive Isotope – The nucleus is unstable and breaks down (decays) emitting rays/particles when the ratio of protons to neutrons moves away from 1: 1. • Natural Transmutation (Table N) – Occurs in nature. • Artificial Transmutation – not radioactive, use a ray/particle and bombard to make the isotope radioactive.
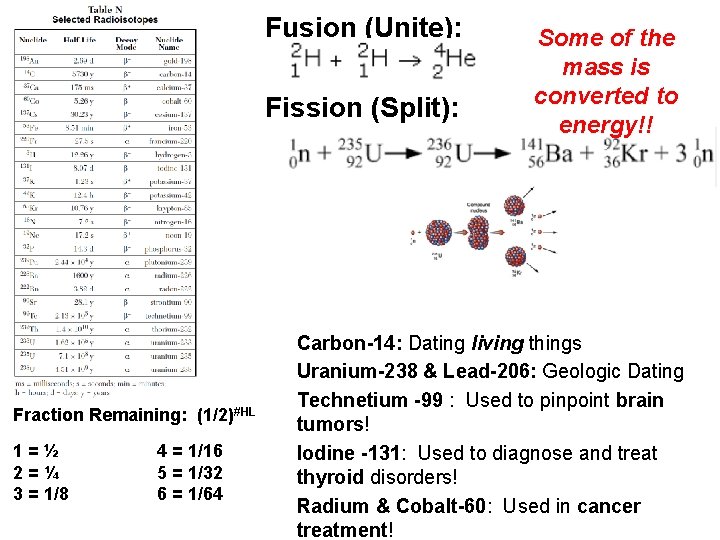
Fusion (Unite): Fission (Split): Fraction Remaining: (1/2)#HL 1=½ 2=¼ 3 = 1/8 4 = 1/16 5 = 1/32 6 = 1/64 Some of the mass is converted to energy!! Carbon-14: Dating living things Uranium-238 & Lead-206: Geologic Dating Technetium -99 : Used to pinpoint brain tumors! Iodine -131: Used to diagnose and treat thyroid disorders! Radium & Cobalt-60: Used in cancer treatment!
- Slides: 31