p H Ch 15 Objectives Describe the selfionization

p. H Ch 15

Objectives • Describe the self-ionization of water. • Define p. H, and give the p. H of a neutral solution at 25 o. C. • Explain and use the p. H scale.
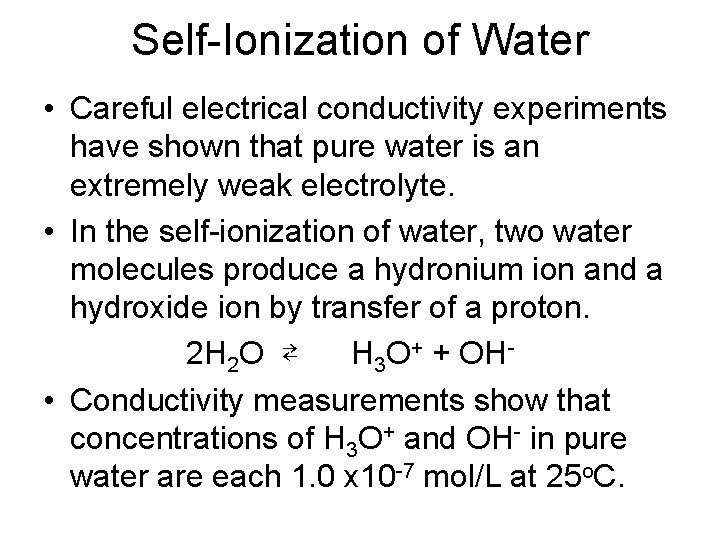
Self-Ionization of Water • Careful electrical conductivity experiments have shown that pure water is an extremely weak electrolyte. • In the self-ionization of water, two water molecules produce a hydronium ion and a hydroxide ion by transfer of a proton. 2 H 2 O ⇄ H 3 O+ + OH • Conductivity measurements show that concentrations of H 3 O+ and OH- in pure water are each 1. 0 x 10 -7 mol/L at 25 o. C.
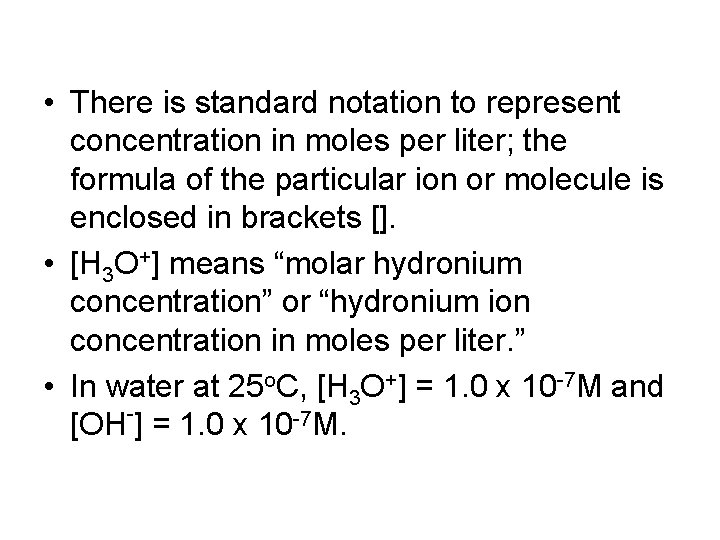
• There is standard notation to represent concentration in moles per liter; the formula of the particular ion or molecule is enclosed in brackets []. • [H 3 O+] means “molar hydronium concentration” or “hydronium ion concentration in moles per liter. ” • In water at 25 o. C, [H 3 O+] = 1. 0 x 10 -7 M and [OH-] = 1. 0 x 10 -7 M.
![• The mathematical product of [H 3 O+] and [OH-] remains constant in • The mathematical product of [H 3 O+] and [OH-] remains constant in](http://slidetodoc.com/presentation_image_h/2c2bcea4e283065b1c412dc0d6b625e0/image-5.jpg)
• The mathematical product of [H 3 O+] and [OH-] remains constant in water and dilute aqueous solutions at constant temperature. • This constant product is called the ionization constant of water, Kw. Kw = [H 3 O+][OH-] = (1. 0 x 10 -7 M ) = 1. 0 x 10 -14 M 2 • The ionization of water increases as temperature increases. Therefore the ion product Kw also increases as temperature increases.
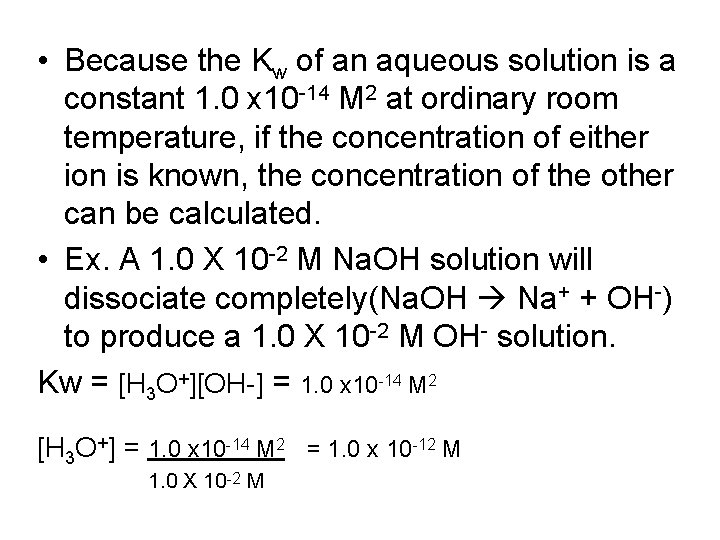
• Because the Kw of an aqueous solution is a constant 1. 0 x 10 -14 M 2 at ordinary room temperature, if the concentration of either ion is known, the concentration of the other can be calculated. • Ex. A 1. 0 X 10 -2 M Na. OH solution will dissociate completely(Na. OH Na+ + OH-) to produce a 1. 0 X 10 -2 M OH- solution. Kw = [H 3 O+][OH-] = 1. 0 x 10 -14 M 2 [H 3 O+] = 1. 0 x 10 -14 M 2 = 1. 0 x 10 -12 M 1. 0 X 10 -2 M
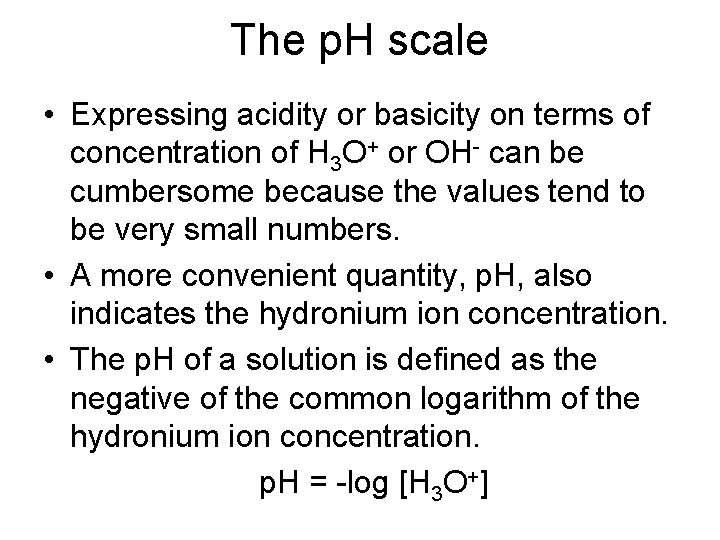
The p. H scale • Expressing acidity or basicity on terms of concentration of H 3 O+ or OH- can be cumbersome because the values tend to be very small numbers. • A more convenient quantity, p. H, also indicates the hydronium ion concentration. • The p. H of a solution is defined as the negative of the common logarithm of the hydronium ion concentration. p. H = -log [H 3 O+]
• Because hydronium ion and hydroxide ion concentrations are the same in pure water, it is neutral. p. H = -log [H 3 O+] = -log (1. 0 x 10 -7 M) = 7 • Any solution in which [H 3 O+] = [OH-] is also neutral. p. OH = 14. 0 - p. H • Solutions in which the [H 3 O+] is greater that the [OH-] are acidic. p. H<7. 0 • Solutions in which the [OH-] is greater than the [H 3 O+] is basic. p. H >7. 0
- Slides: 8