Ozone Bonding Antibonding Nonbonding PrenticeHall 2002 4 electrons
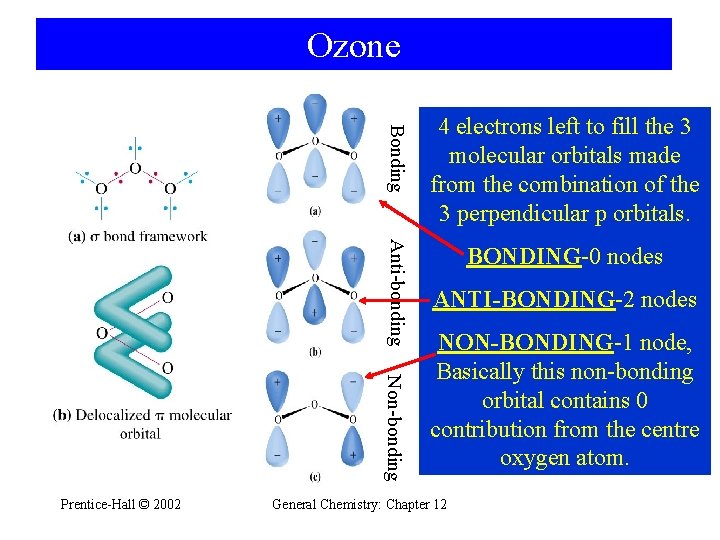
Ozone Bonding Anti-bonding Non-bonding Prentice-Hall © 2002 4 electrons left to fill the 3 molecular orbitals made from the combination of the 3 perpendicular p orbitals. BONDING-0 nodes ANTI-BONDING-2 nodes NON-BONDING-1 node, Basically this non-bonding orbital contains 0 contribution from the centre oxygen atom. General Chemistry: Chapter 12
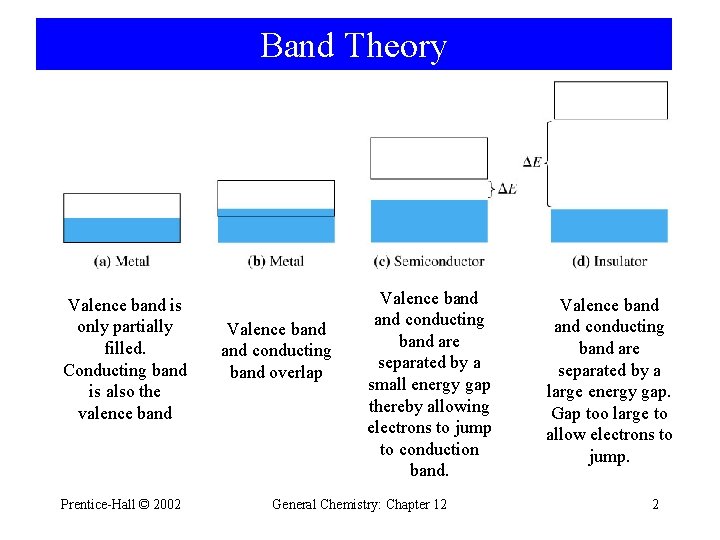
Band Theory Valence band is only partially filled. Conducting band is also the valence band Prentice-Hall © 2002 Valence band conducting band overlap Valence band conducting band are separated by a small energy gap thereby allowing electrons to jump to conduction band. General Chemistry: Chapter 12 Valence band conducting band are separated by a large energy gap. Gap too large to allow electrons to jump. 2
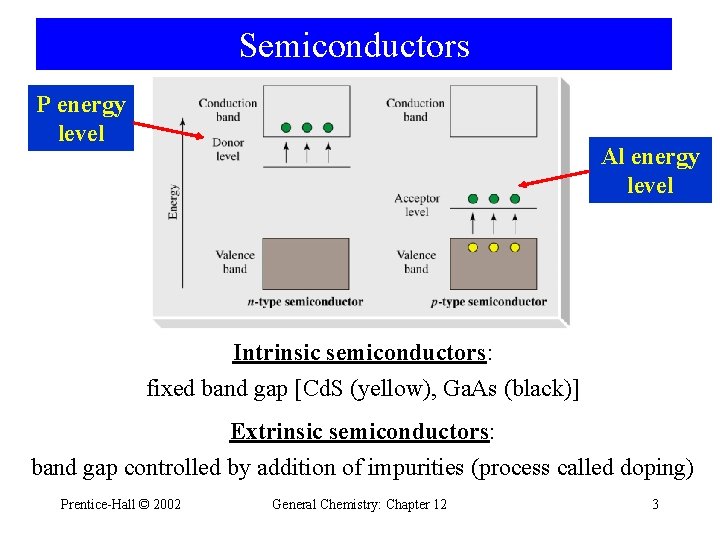
Semiconductors P energy level Al energy level Intrinsic semiconductors: fixed band gap [Cd. S (yellow), Ga. As (black)] Extrinsic semiconductors: band gap controlled by addition of impurities (process called doping) Prentice-Hall © 2002 General Chemistry: Chapter 12 3

Chapter 12 Questions 1, 3, 8, 10, 16, 17, 18, 29, 33, 39, 40, 45, 59, 68, 72, 76 Prentice-Hall © 2002 General Chemistry: Chapter 12 4

General Chemistry Principles and Modern Applications Petrucci • Harwood • Herring 8 th Edition Chapter 13: Liquids, Solids and Intermolecular Forces Philip Dutton University of Windsor, Canada N 9 B 3 P 4 Prentice-Hall © 2002 (modified 2003 by Dr. Paul Root and 2005 by Dr. David Tramontozzi) Prentice-Hall © 2002 General Chemistry: Chapter 12 5

Contents 13 -1 13 -2 13 -3 13 -4 13 -5 13 -6 13 -7 13 -8 Intermolecular Forces and some Properties of Liquids Vaporization of Liquids: Vapor Pressure Some Properties of Solids Phase Diagrams Van der Waals Forces Hydrogen Bonding Chemical Bonds as Intermolecular Forces Crystal structures Energy Changes in the Formation of Ionic Crystals Focus on Liquid Crystals Prentice-Hall © 2002 General Chemistry: Chapter 12 6
13 -1 Intermolecular Forces and Some Properties of Liquids • Cohesive Forces – Intermolecular forces between like molecules. • Adhesive Forces – Intermolecular forces between unlike molecules. • Surface Tension – Energy or work required to increase the surface area of a liquid. • Viscosity – A liquids resistance to flow Prentice-Hall © 2002 General Chemistry: Chapter 12 7
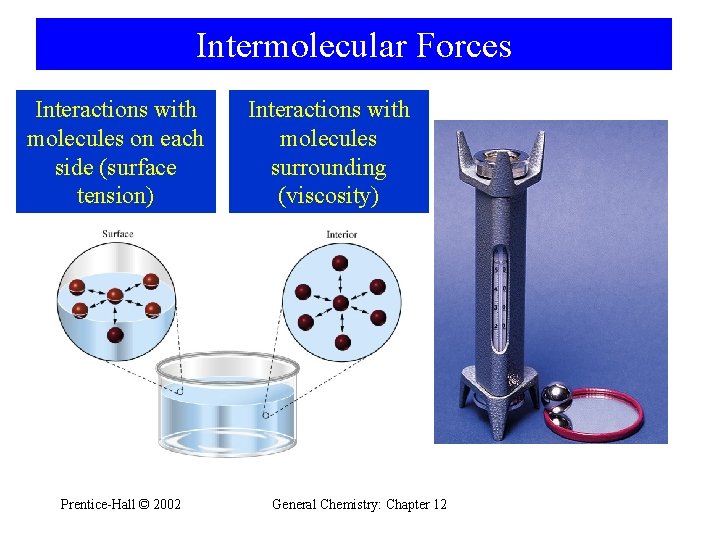
Intermolecular Forces Interactions with molecules on each side (surface tension) Prentice-Hall © 2002 Interactions with molecules surrounding (viscosity) General Chemistry: Chapter 12

Intermolecular Forces Clean glass Oil covered glass Capillary action Prentice-Hall © 2002 Adhesive forces between water and glass cause meniscus to form. Hg metallic bonds are stronger than adhesive forces with glass therefore no meniscus forms. General Chemistry: Chapter 12 9
- Slides: 9