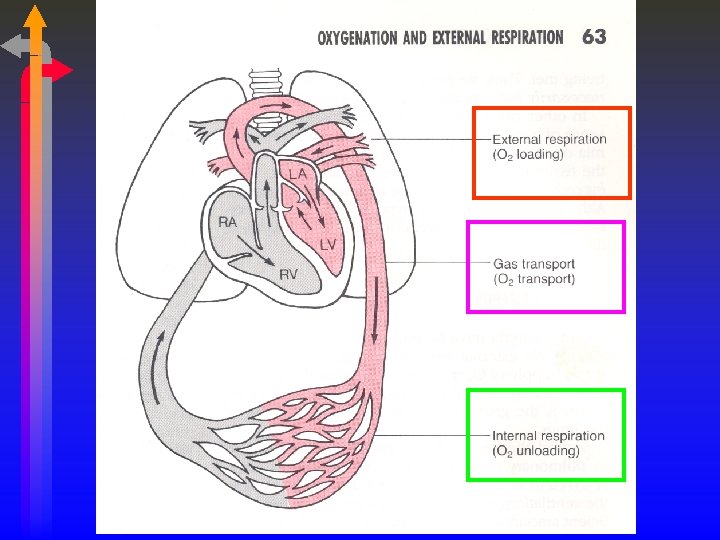
Oxygen Transport and Internal Respiration Module E Chapter

















- Slides: 33

Oxygen Transport and Internal Respiration Module E Chapter 7 Malley

Objectives • State the formula for calculating oxygen content. • Given a Pa. O 2, calculate the amount of oxygen that is dissolved in plasma in vol%. • Describe the chemical structure of hemoglobin. • Describe the relationship between oxygen and hemoglobin in the presence of various factors. • List the abnormal species of hemoglobin. • Describe how hypoxia can result from ineffective cellular utilization of oxygen.


Oxygen Transport • TWO WAYS TO CARRY OXYGEN • DISSOLVED IN PLASMA • COMBINED WITH HEMOGLOBIN • THE TOTAL AMOUNT OF OXYGEN PRESENT IS CALLED THE OXYGEN CONTENT. • ANY QUESTIONS?


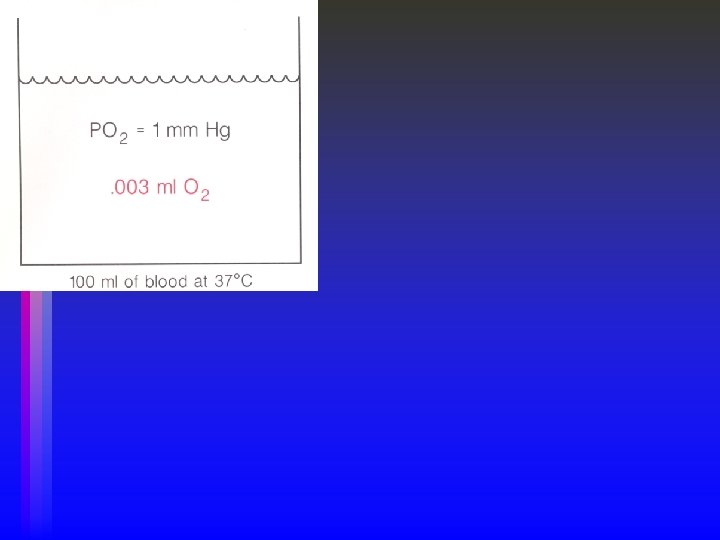
Dissolved Oxygen • Diffusion occurs because of a partial pressure difference between alveolus and capillary (gas to liquid). • The volume of gas that dissolves depends on the Solubility coefficient of the gas. • Higher solubility, more dissolved, regardless of partial pressure. • O 2 dissolved in blood @ 37° C is. 003 m. L O 2/100 m. L/mm Hg or • A normal individual will dissolve between 0. 24 and 0. 3 m. L of oxygen/m. L of blood at one atmosphere and 37° C. • Dissolved O 2 (Pa. O 2) represents 0. 3 vol% • Linear relationship between Pa. O 2 and the volume of oxygen dissolved • 0. 3 vol% at 100 mm. Hg Pa. O 2, 0. 6 vol% at 200 mm. Hg Pa. O 2


Dissolved Oxygen – Pa. O 2 • Pa. O 2 does not tell us how much oxygen is in the blood. • Tells us about the potential driving pressure at a later point, but the volume (vol%) is the key issue. • Determined by PAO 2, �/� relationships, level of ventilation and the AC membrane. • THINK ABOUT THE CAUSES OF HYPOXEMIA!

Combined O 2 • Dissolved oxygen is inadequate for metabolic needs. • Need a substance which will bind to oxygen and carry it, BUT will release it when needed. • Voila! Hemoglobin! • Miracle #2…Hemoglobin also carries carbon dioxide and is a key buffer of [H+].

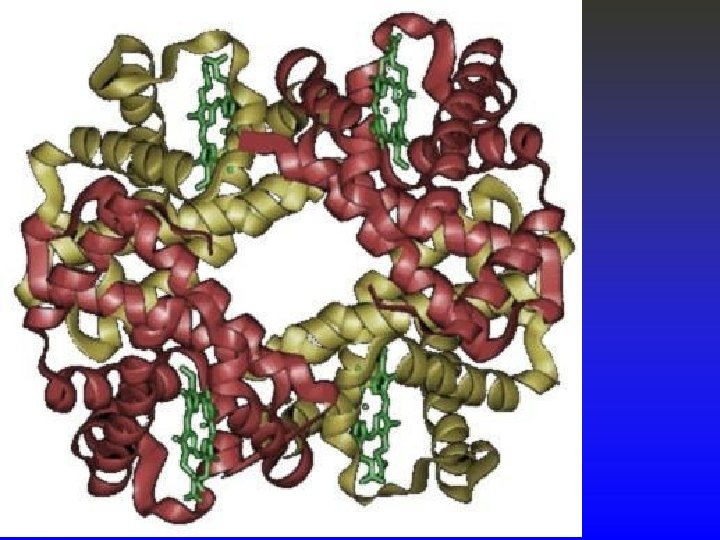
Hemoglobin • Hemoglobin is composed of two groups: • Heme Group – Primarily iron in the Fe+2 (ferrous) state. • Globulin – 4 amino acid chains; • 2 alpha chains made up of 141 amino acids, • 2 beta chains made up of 146 amino acids. • Large molecule – GMW of 64, 500 g. • One heme group binds with one of the amino acid chains and oxygen binds with the iron in the heme group in a reversible way. O 2 + Fe+2 « Fe. O 2


Hemoglobin & the RBC • “Normal” hemoglobin level • 15 g/dl (men) & 13 -14 g/dl (women) - Malley • 15 + 1. 5 g/dl (men) & 13. 5 + 1. 5 g/dl (women) – Egan • 15 + 2 (men) & 14 + 2 (women) - Easy • Why do women have less? • The hemoglobin molecule resides in the erythrocyte and is responsible for giving the blood its red color. • Hundreds of hemoglobin variants. • Normal: A, A 2, F • Abnormal: S, H

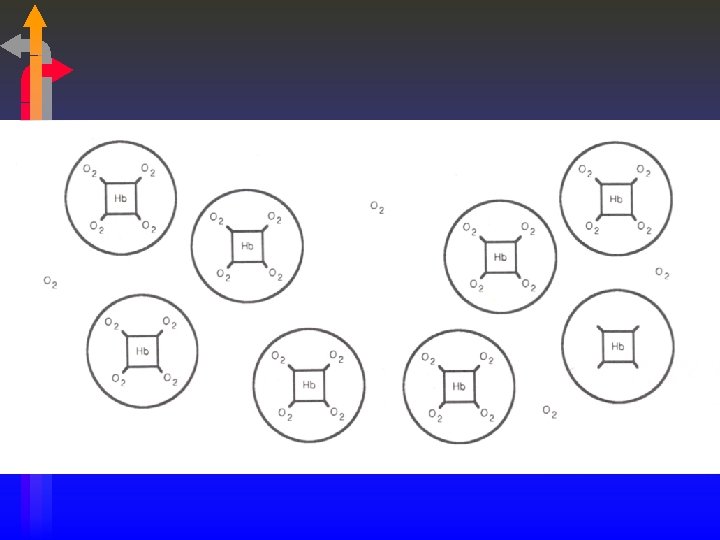
Combined Oxygen • The affinity of hemoglobin for oxygen increases with each oxygen molecule attached • “All or Nothing” • Each gram of Hemoglobin combines with 1. 34 m. L of oxygen. • Oxygen Saturation only talks about how much hemoglobin is saturated, NOT how much hemoglobin is present.


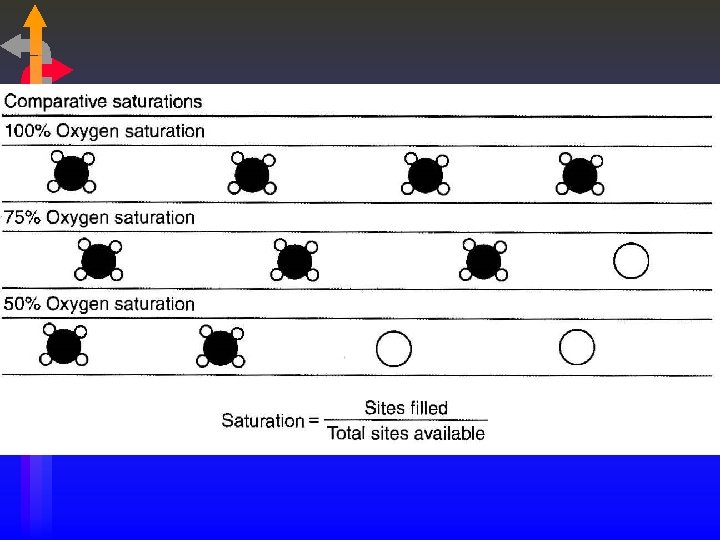
Oxygen Saturation • Saturation = Sites Filled Total Sites Available • Example: • 80 sites filled = 80% saturation 100 sites available


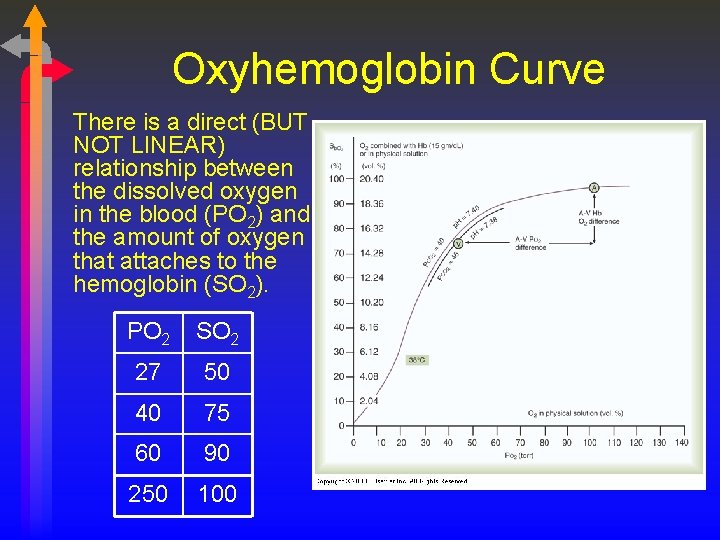
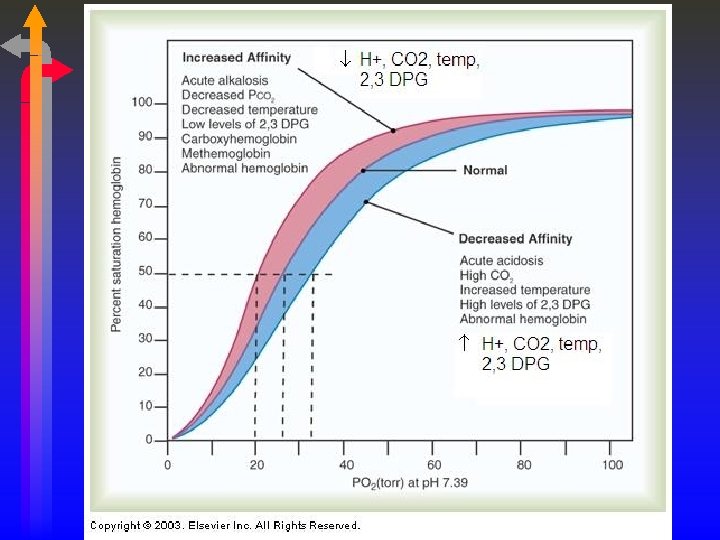
Oxyhemoglobin Curve There is a direct (BUT NOT LINEAR) relationship between the dissolved oxygen in the blood (PO 2) and the amount of oxygen that attaches to the hemoglobin (SO 2). PO 2 SO 2 27 50 40 75 60 90 250 100

Significance of OHDC Shape • The steep line signifies that a small change in PO 2 will yield a large change in SO 2. • This is the dissociation portion of the curve. • Oxygen release is maximized. • A large amount of oxygen can be delivered to the tissues in times of compromised cardiovascular function or increased demands. • COPD patients “live” on the steep portion, small changes in Pa. O 2 will yield greater changes in Sa. O 2. • The flat line signifies that a large change in PO 2 results in only a minimal increase in SO 2. • This is the association portion of the curve. • Oxygen carrying is maximized. • The PO 2 has to fall below 60 mm Hg before saturation becomes significantly affected. • Aging, High Altitudes

P 50 • The specific affinity of oxygen for hemoglobin can be assessed by evaluating at what PO 2 there is a 50% SO 2. • It is measured at 37° C, a Pa. CO 2 40 mm Hg, p. H 7. 40. • Normal value is 27 mm Hg (25 -27 mm Hg) • As P 50 changes, we say the “curve has shifted”.

Shifts in the OHDC • Left Shift • • Increased oxygen affinity for hemoglobin. This is the expected shift at the lungs (Left-Lungs) For any given PO 2, SO 2 will be higher. Decreasing P 50 • Right Shift • • Decreased oxygen affinity for hemoglobin. This is the expected shift at the tissues. For any given PO 2, SO 2 will be lower. Increasing P 50


Bohr Effect • The effect of CO 2 on the OHDC is known as the Bohr Effect. (OH – Bohr) • High PCO 2 levels and low p. H decrease affinity of hemoglobin for oxygen (a right-ward shift) • This occurs at the tissues where a high level of PCO 2 and acidemia contribute to the unloading of oxygen (and the attachment of CO 2 to the unbound hemoglobin). • At the lungs, the high levels of oxygen facilitate the release of CO 2 from the Hemoglobin molecule. This is known as the Haldane Effect.

2, 3 DPG • 2, 3 DPG is an organic phosphate normally found in RBC, that has a tendency to bind with Hemoglobin and thereby decrease the affinity of Hemoglobin for oxygen. • It promotes a rightward shift and enhances oxygen unloading at the tissues. This shift is longer in duration that due to [H+], PCO 2 or temperature. • A doubling of DPG will result in a 10 torr increase in P 50. • The levels increase with cellular hypoxia. • • Anemia Hypoxemia secondary to COPD. Congenital Heart Disease Ascent to high altitudes • The levels are reduced with • Septic Shock • Acidemia • STORED BLOOD in ACD: NO DPG after 2 weeks of storage.

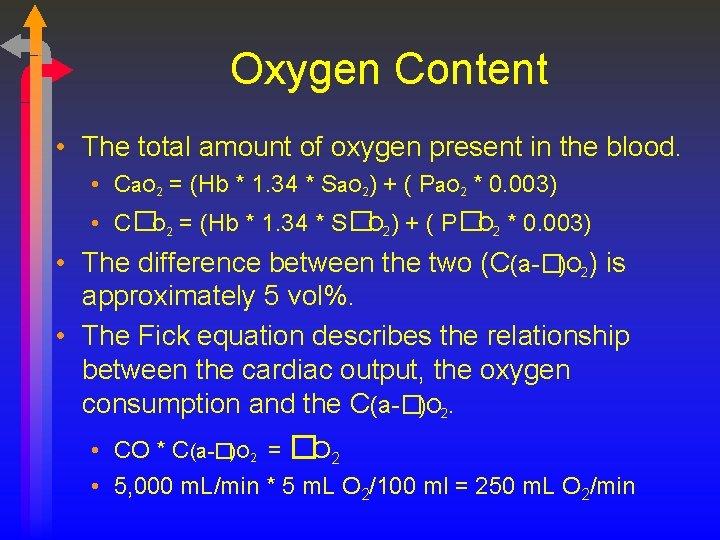
Oxygen Content • The total amount of oxygen present in the blood. • Ca. O 2 = (Hb * 1. 34 * Sa. O 2) + ( Pa. O 2 * 0. 003) • C�O 2 = (Hb * 1. 34 * S�O 2) + ( P�O 2 * 0. 003) • The difference between the two (C(a-�)O 2) is approximately 5 vol%. • The Fick equation describes the relationship between the cardiac output, the oxygen consumption and the C(a-�)O 2. • CO * C(a-�)O 2 = �O 2 • 5, 000 m. L/min * 5 m. L O 2/100 ml = 250 m. L O 2/min

Cyanosis • A clinical condition manifested by a bluish discoloration of the mucous membranes or nail beds. • Peripheral vs. Central • Present when 5 g/dl of hemoglobin is desaturated. • This usually correlates with a Sa. O 2 below 85%. • Calculated as follows: [(Hb * Arterial Desaturation) + (Hb * Venous Desaturation)] /2 • Normal Arterial Desaturation is 100% - 98% or 2%. • Normal Venous Desaturation is 100% - 75% or 25%. [(15 *. 02) + (15 *. 25)] /2 = [. 3 +3. 75] / 2 = 4. 05/2 = 2. 025 g% • ANEMIC PATIENTS WILL NEVER BE CYANOTIC!

Oxygen Transport • The volume of oxygen leaving the left ventricle each minute. • Ca. O 2 * CO = 20 ml O 2/100 m. L * 5, 000 m. L/min • 1, 000 m. L O 2 / min • If oxygen consumption is 250 m. L O 2 / min, then we “extract” 25% of the total. • This can also be determined by dividing the C(a-�)O 2 by the Ca. O 2. (5 vol% / 20 vol%) =. 25 • Key factors are the Pa. O 2, SV, & HR

Hemoglobin Abnormalities • • • Carboxyhemoglobin Sulfhemoglobin Fetal Hemoglobin Methemoglobin Hemoglobin S

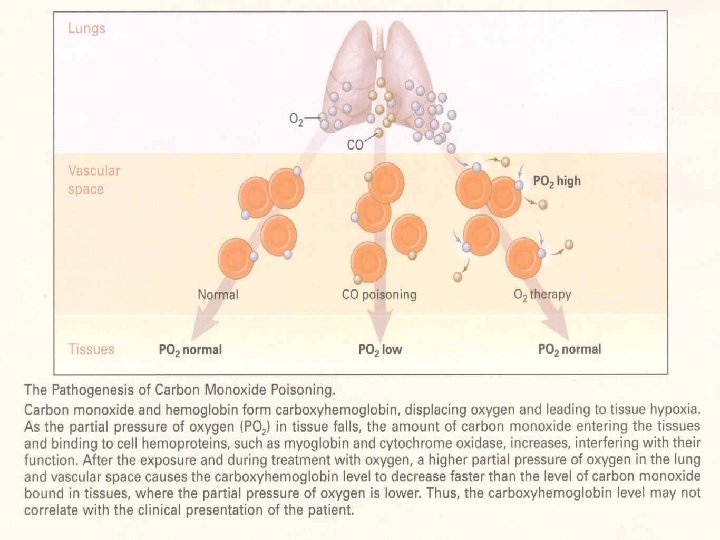
Carboxyhemoglobin • Carbon Monoxide has 245 times the affinity for hemoglobin as oxygen. • Causes a leftward shift (increased affinity of Oxygen) • Normal level is less than 3% • 10% is common with smokers. • Toxic at 20%; Lethal at 50%. • Venous Hb. CO = Arterial Hb. CO (Ann Emerg Med. 1995 Apr; 25(4): 481 -3) • Suspect incomplete combustion if level is elevated. • Furnace, space heater • Treatment is 100% oxygen • Reduces half-life of Hb. CO from 5 hours to 20 minutes. • Hyperbaric treatment remains controversial.


Methemoglobin • • A normal variant of adult hemoglobin. Ferrous to Ferric (loses an electron, Fe+3) Normal levels are less than 1%. Usually associated with excessive nitrate ingestion. • • Amyl Nitrate Nitroglycerine Nipride Topical anesthetics • Blood will appear rusty or brown in color. • Treatment with methylene blue

Sulfhemoglobin • Sulfhemoglobin results from the union of hemoglobin with medications such as phenacetin (sedative no longer used) or sulfonamides (antibiotics including Bactrim & Septra). • The resultant form of hemoglobin is unable to transport oxygen, and is untreatable. • The only treatment is to wait until the affected red blood cells are destroyed as part of their normal life cycle.

Fetal Hemoglobin (Hb. F) • Fetal hemoglobin (hemoglobin F) is the main hemoglobin that transports oxygen around the body of the developing baby during the last 7 months of pregnancy. • It has a greater affinity for oxygen than Hemoglobin A (P 50 of 20 mm Hg). • At about 30 weeks gestation, the fetus begins to make increasing amounts of hemoglobin A. • Hemoglobin F does not turn into hemoglobin A. • As they grow babies automatically turn off the production of hemoglobin F (usually complete by one year). Failure to stop Hemoglobin F production is found in certain beta thalassemias. Possible link to SIDS.

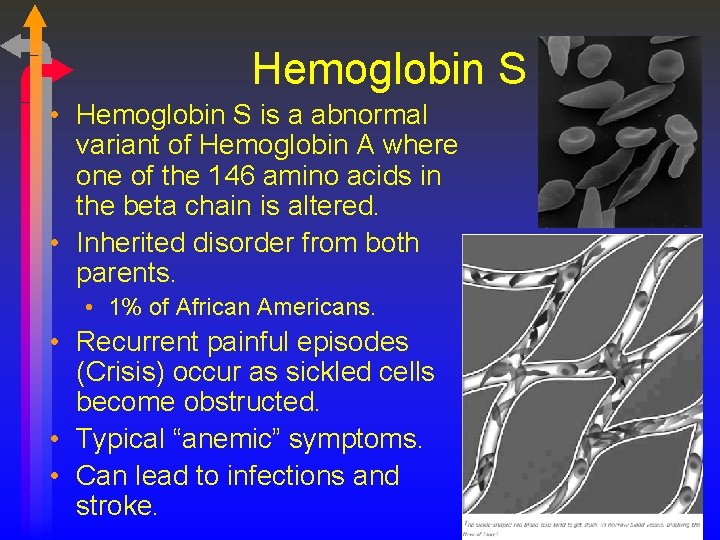
Hemoglobin S • Hemoglobin S is a abnormal variant of Hemoglobin A where one of the 146 amino acids in the beta chain is altered. • Inherited disorder from both parents. • 1% of African Americans. • Recurrent painful episodes (Crisis) occur as sickled cells become obstructed. • Typical “anemic” symptoms. • Can lead to infections and stroke.