OXYGEN TOXICITY Prepared and presented by Dr Debora

OXYGEN TOXICITY Prepared and presented by: Dr Debora, Dr. Gibonce-DEPARTMENT OF ANAESTHESIOLOGY AND ICU BMC 19 th of May, 2016.

OXYGEN TOXICITY OUTLINE a)Introduction b)Functions/Indications c)Measurements of O 2 d)Mechanisms of O 2 toxicity e)Protective mechanisms against Reactive Oxygen Species(ROS). g)Complications of Oxygen toxicity h)Take home message
INTRODUCTION Oxygen is a chemical element with symbol O and atomic number 8. By mass, oxygen is the third-most abundant element in the universe, after hydrogen and helium, comprising about 21% of atmospheric air. OXYGEN IS A DRUG AND IS PRESCRIBED!! Oxygen therapy is the administration of O 2 at a concentration greater than a room air(21%) with a goal of treating/preventing symptoms and manifestations of hypoxia.

MEDICAL OXYGEN SOURCE The sources of medical O 2 are: 1)Oxygen Cylinders. 2)Oxygen concentrators. 3) oxygen plant.

USES OF MEDICAL OXYGEN The aerobic metabolic system functions using the Krebs Cycle, a complex series of chemical reactions that use oxygen to convert nutrients (carbohydrates, fats, and protein) to carbon dioxide and adenosine triphosphate (ATP), an energy-rich compound.

INDICATIONS q The main indication of O 2 thearpy is treatment/prevention of Hypoxia(i. e state of low O 2 concentration reaching the tissues).

7 09 -Mar-21 CAUSES OF HYPOXIA PO 2 in air Inadequate alveolar ventilation Impaired O 2 uptake Inadequate blood flow to tissues Poisoning or inadequate cellular enzymes q. Reduced partial pressure of oxygen in air. q. Inadequate alveolar ventilation. q. Impaired pulmonary uptake.
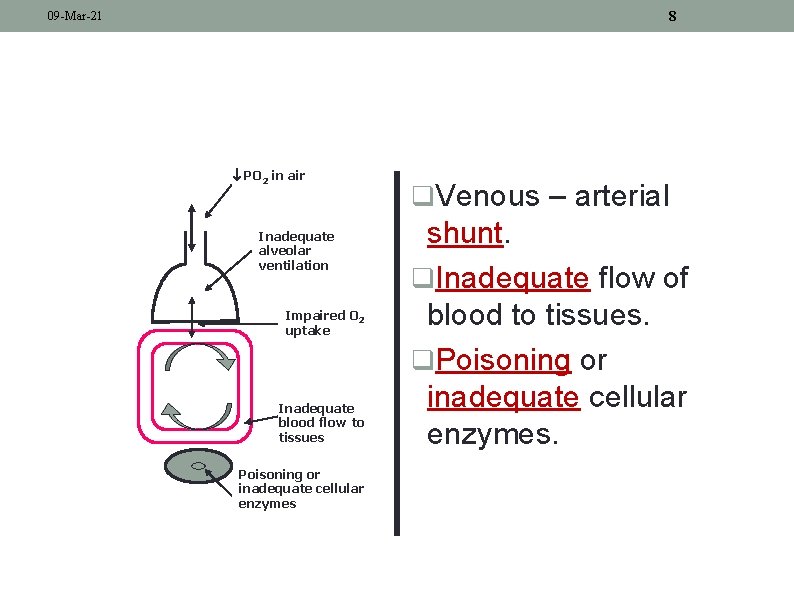
8 09 -Mar-21 PO 2 in air Inadequate alveolar ventilation Impaired O 2 uptake Inadequate blood flow to tissues Poisoning or inadequate cellular enzymes q. Venous – arterial shunt. q. Inadequate flow of blood to tissues. q. Poisoning or inadequate cellular enzymes.

SIGNS OF HYPOXIA Generally the pt will present with signs of respiratory distress which include: Dyspnea. Tachypnea. Severe hypoxaemia (decreased oxygen concentration in the blood) Pulmonary hypertension. Cyanosis, etc.
MEASUREMENTS OF O 2 LEVELS IN THE BODY The most common methods used are: a) Pulse oxymeter. b) Arterial blood gases analysis(ABG). q ABG is more accurate than Pulse oxymeter because it measures the actual concentration of O 2 in the blood in relation to other blood gases. q Factors hindering P/oxymeter accuracy are: -poor peripheral blood flow(i. e hypotension), coldness, nail polish, etc.


Need of O 2 is determined by: • Clinical evaluation plus measured O 2 level • By- Pulse oxymeter or ABG • Generally agreed indication for O 2 therapy-Persistent SPO 2<90%.

OXYGEN TOXICITY Not only is too little oxygen a problem, too much is as well. Defn: q Oxygen toxicity is a condition resulting from the harmful effects of breathing molecular oxygen (O 2) at increased partial pressures. q

Exposure time matters alot… • Oxygen toxicity – can occur with Fio 2 > 60% longer than 36 hrs • Fio 2>80%longer than 24 hrs • Fio 2>100%longer than 12 hrs

MECHANISM OF OXYGEN TOXICITY Usually Reactive Oxygen Species (ROS) are produced during normal physiological processes like Electron Transport Chain(ETC), etc. The most commonly produced ROS are: -Superoxide anion (O 2 -) -Hydroxyl radical (OH • ) -Hydrogen peroxide (H 2 O 2) -Hypochlorous acid (HOCl )

ELECTRON TRANSPORT CHAIN

Under normal circumstances the body is able to handle the ROS produced using anti oxidants but can be overwhelmed incase of excessive production of ROS, hence toxic effects of O 2. Glutathione is the mother of all anti oxidants. Others Anti-oxidants are catalase, superoxide dismutase, vitamin. C, E.

SOURCES OF ROS Reactive Oxygen Species (ROS) are a natural occurrence: q. Accidental products of nonenzymatic and enzymatic processes. q. Deliberate production by immune cells killing pathogens. q. UV irradiation, radioactive chemicals, Xrays ü
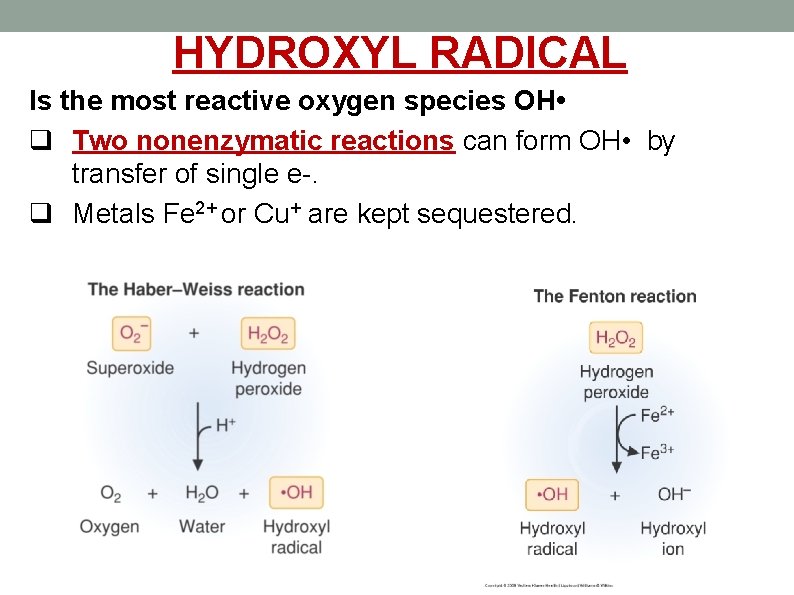
HYDROXYL RADICAL Is the most reactive oxygen species OH • q Two nonenzymatic reactions can form OH • by transfer of single e-. q Metals Fe 2+ or Cu+ are kept sequestered.

Harmful effects of these radicals… Oxygen radicals react with cell components: • • Lipid peroxidation of membranes. Increased permeability → influx Ca 2+ → mitochondrial damage. Proteins oxidized and degraded. DNA oxidized → breakage.
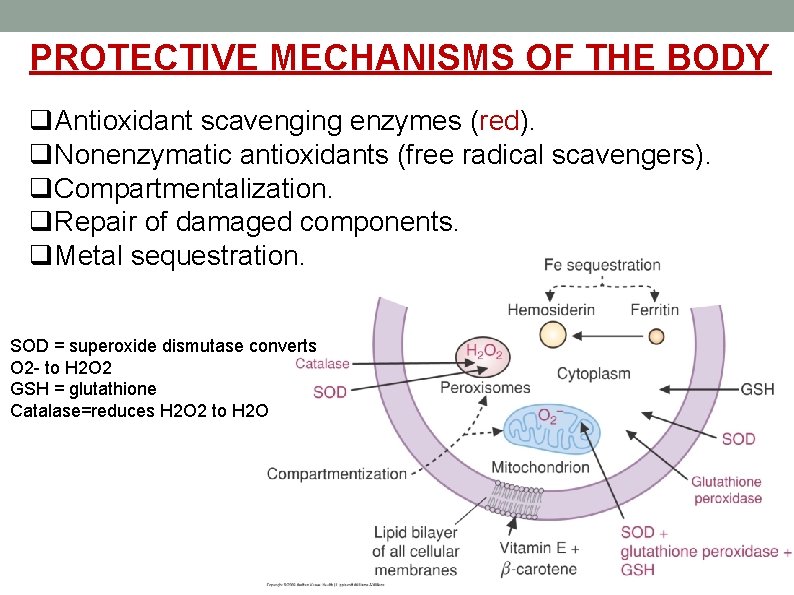
PROTECTIVE MECHANISMS OF THE BODY q. Antioxidant scavenging enzymes (red). q. Nonenzymatic antioxidants (free radical scavengers). q. Compartmentalization. q. Repair of damaged components. q. Metal sequestration. SOD = superoxide dismutase converts O 2 - to H 2 O 2 GSH = glutathione Catalase=reduces H 2 O 2 to H 2 O

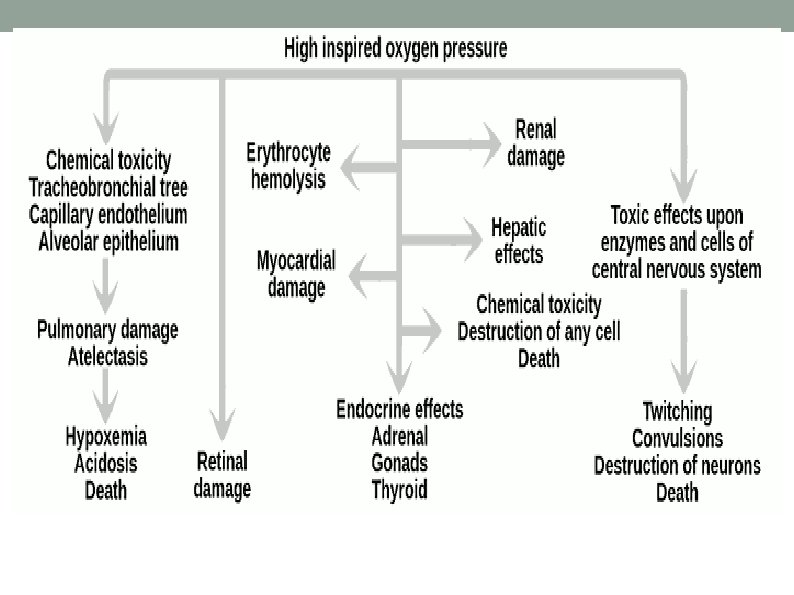
COMPLICATIONS OF OXYGEN TOXICITY The common affected sytems/organs are: a) CNS - Convulsions(tonic–clonic seizure). b) Pulmonary - Atelectasis. c) Eyes - Retinal damage. q

TAKE HOME MESSAGE We need to emphasize on good use of O 2 due to the following reasons: a) Detrimental effects of unnecessary O 2 therapy. b) Cost effective utilization of O 2. - Very expensive. c) Risk of fire hazards. -Highly inflammable gas. -Needs good and safe storage conditions. q

SUGGESTIONS O 2 therapy should be used only if there are confirmed indications. Causative problem of Hypoxia should be identified and intervened appropriately-giving O 2 alone is not a solution. Use of appropriate P/oxymeter size to age. Close monitoring of the pts on O 2 therapy(i. e O 2 saturation level). Avoid combustive materials close to cylinders, egoil, kerosene, etc.

Refferences: Clinical Anesthesia-Barash. , 7 th ed. Clinical Anesthesia-Morgan and Mikhail. , 5 th ed. Harper's Biochemistry. , 30 th ed. The ICU Book. . , 4 th ed.

THANK YOU
- Slides: 27