Oxygen Storage in Muscle Tissue Myoglobin Mb Originally
Oxygen Storage in Muscle Tissue Myoglobin (Mb) • Originally isolated from sperm whales • 10 X abundance greater in aquatic- than terrestrial-mammals • Mb knockout mice exhibit normal exercise capacity • Detoxification of reactive nitric oxide a signaling molecule
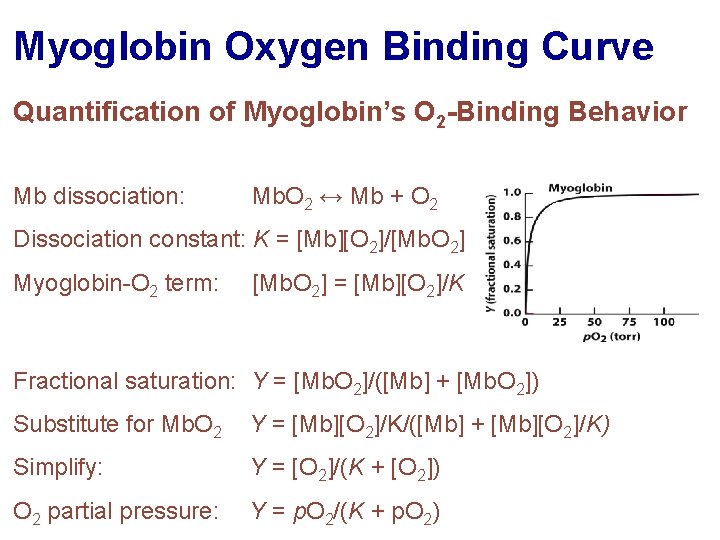
Myoglobin Oxygen Binding Curve Quantification of Myoglobin’s O 2 -Binding Behavior Mb dissociation: Mb. O 2 ↔ Mb + O 2 Dissociation constant: K = [Mb][O 2]/[Mb. O 2] Myoglobin-O 2 term: [Mb. O 2] = [Mb][O 2]/K Fractional saturation: Y = [Mb. O 2]/([Mb] + [Mb. O 2]) Substitute for Mb. O 2 Y = [Mb][O 2]/K/([Mb] + [Mb][O 2]/K) Simplify: Y = [O 2]/(K + [O 2]) O 2 partial pressure: Y = p. O 2/(K + p. O 2)
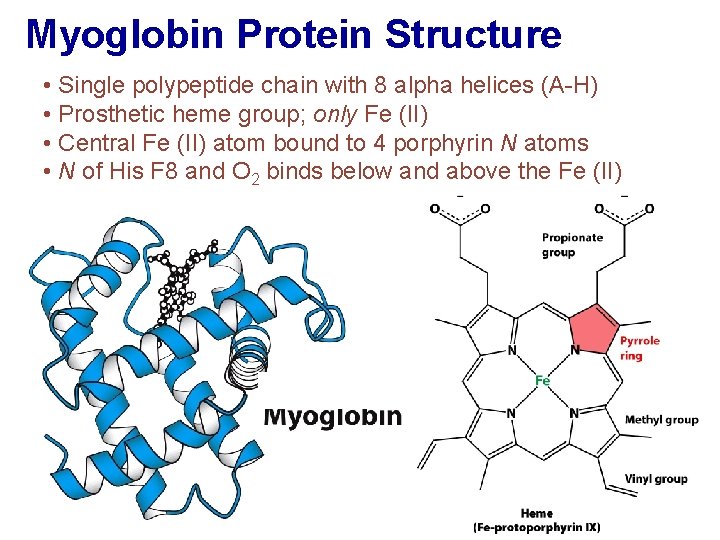
Myoglobin Protein Structure • Single polypeptide chain with 8 alpha helices (A-H) • Prosthetic heme group; only Fe (II) • Central Fe (II) atom bound to 4 porphyrin N atoms • N of His F 8 and O 2 binds below and above the Fe (II)
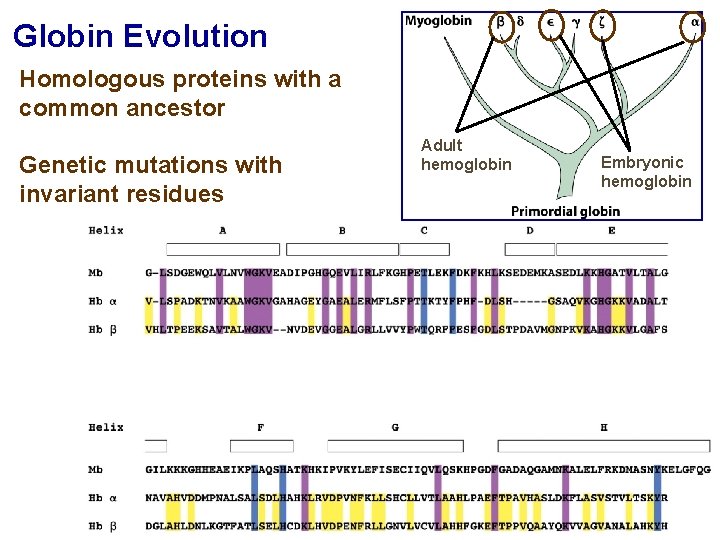
Globin Evolution Homologous proteins with a common ancestor Genetic mutations with invariant residues Adult hemoglobin Embryonic hemoglobin
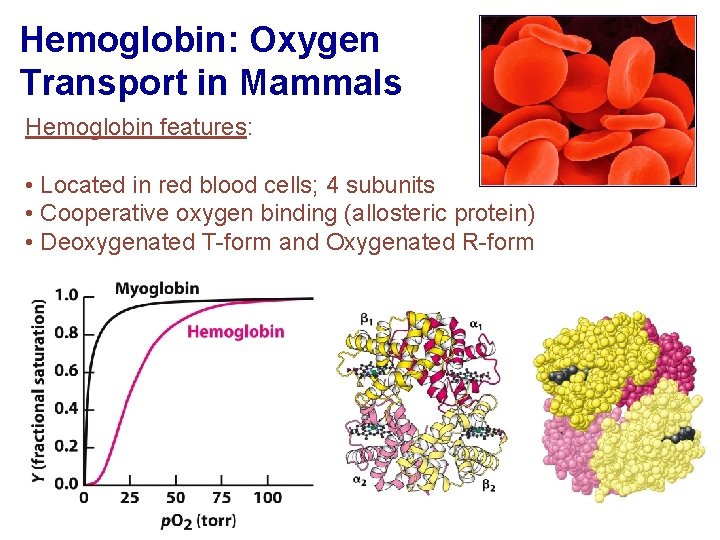
Hemoglobin: Oxygen Transport in Mammals Hemoglobin features: • Located in red blood cells; 4 subunits • Cooperative oxygen binding (allosteric protein) • Deoxygenated T-form and Oxygenated R-form
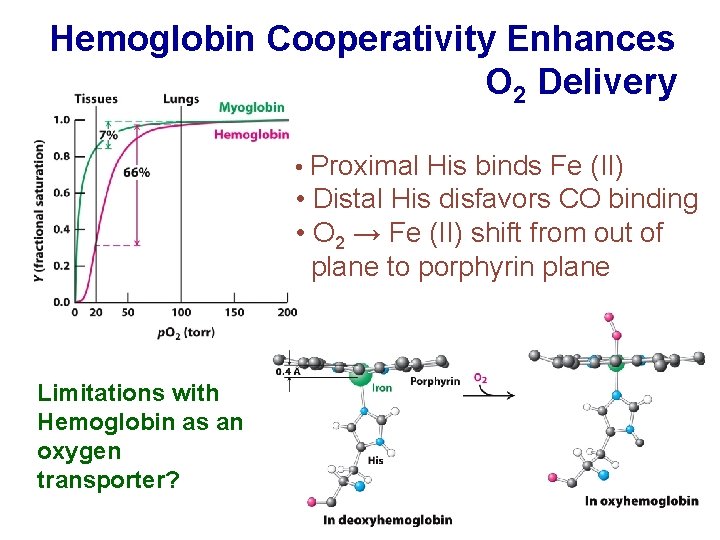
Hemoglobin Cooperativity Enhances O 2 Delivery • Proximal His binds Fe (II) • Distal His disfavors CO binding • O 2 → Fe (II) shift from out of plane to porphyrin plane Limitations with Hemoglobin as an oxygen transporter?
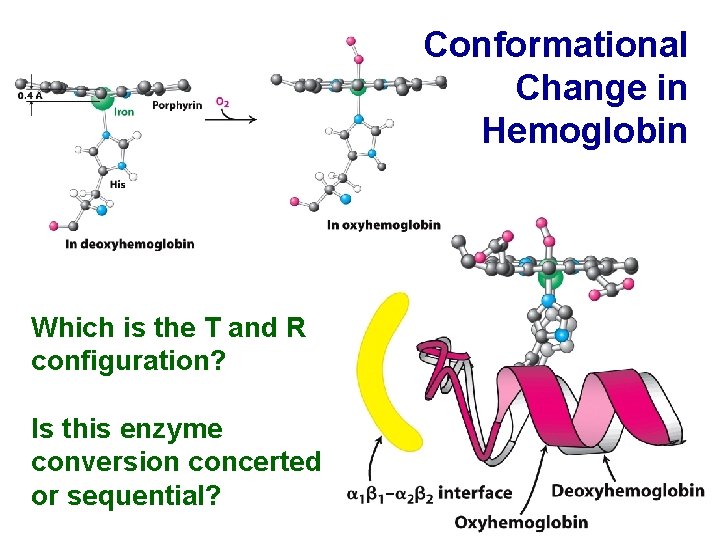
Conformational Change in Hemoglobin Which is the T and R configuration? Is this enzyme conversion concerted or sequential?
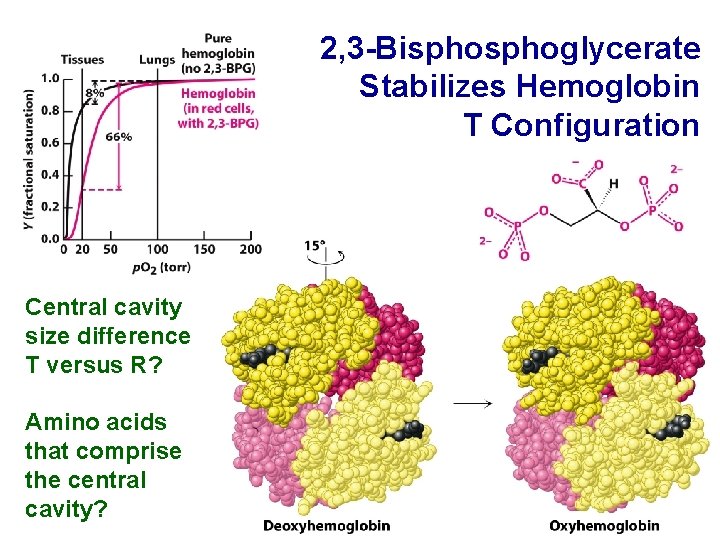
2, 3 -Bisphoglycerate Stabilizes Hemoglobin T Configuration Central cavity size difference T versus R? Amino acids that comprise the central cavity?
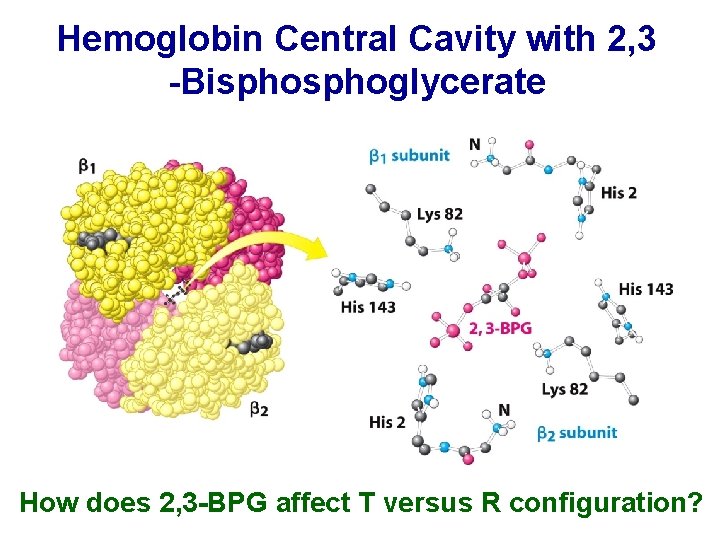
Hemoglobin Central Cavity with 2, 3 -Bisphoglycerate How does 2, 3 -BPG affect T versus R configuration?
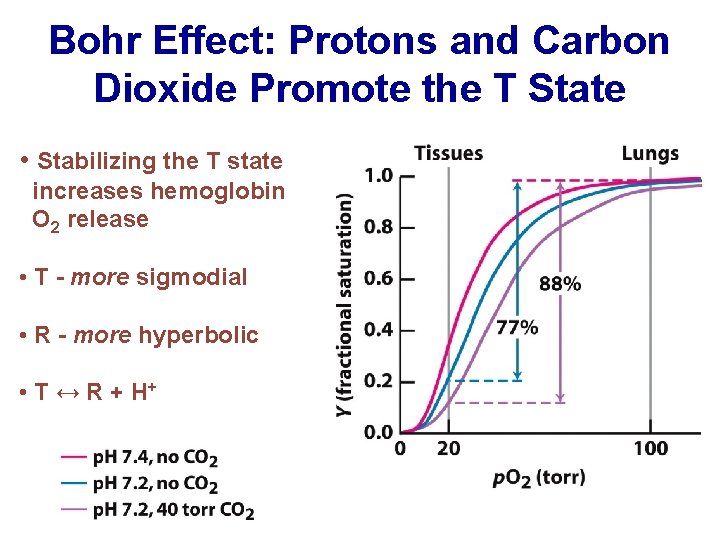
Bohr Effect: Protons and Carbon Dioxide Promote the T State • Stabilizing the T state increases hemoglobin O 2 release • T - more sigmodial • R - more hyperbolic • T ↔ R + H+
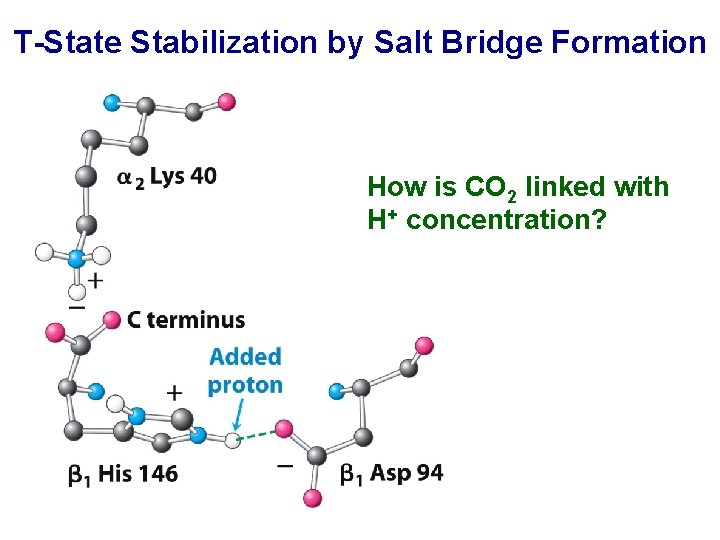
T-State Stabilization by Salt Bridge Formation How is CO 2 linked with H+ concentration?
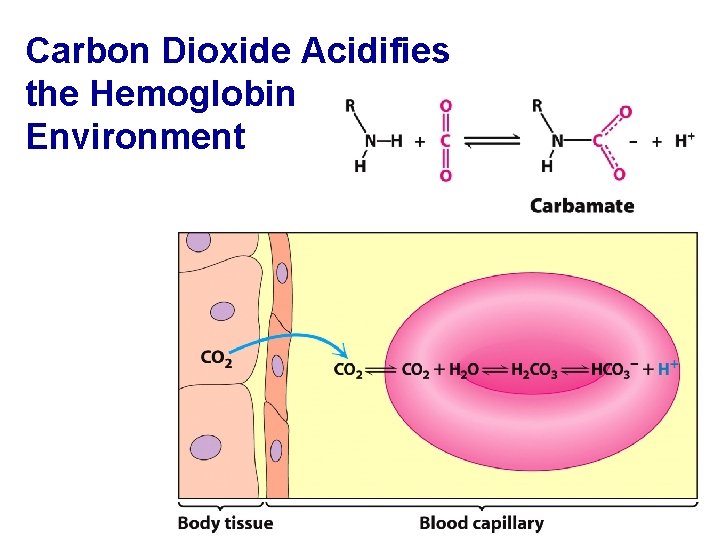
Carbon Dioxide Acidifies the Hemoglobin Environment
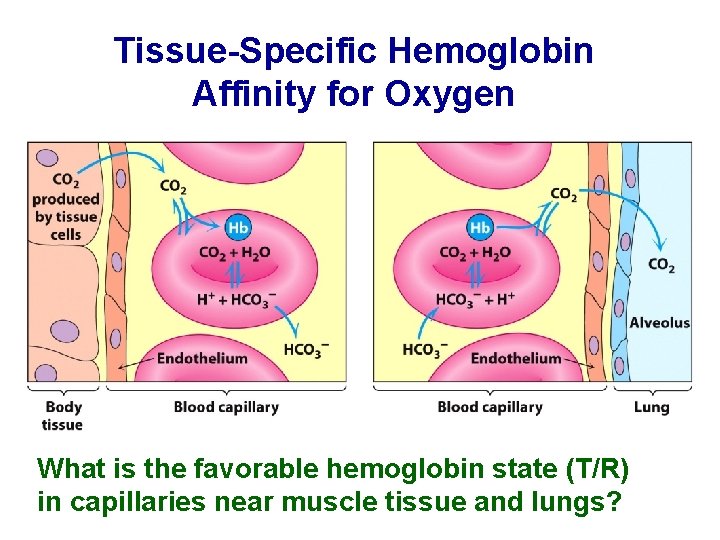
Tissue-Specific Hemoglobin Affinity for Oxygen What is the favorable hemoglobin state (T/R) in capillaries near muscle tissue and lungs?
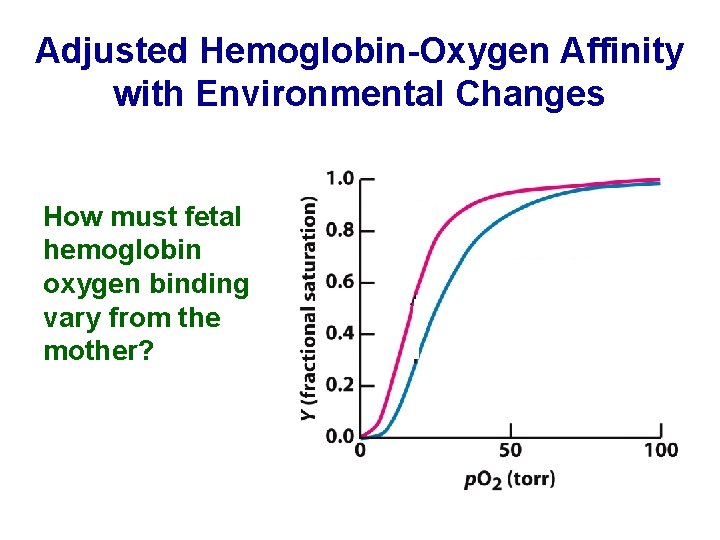
Adjusted Hemoglobin-Oxygen Affinity with Environmental Changes How must fetal hemoglobin oxygen binding vary from the mother?
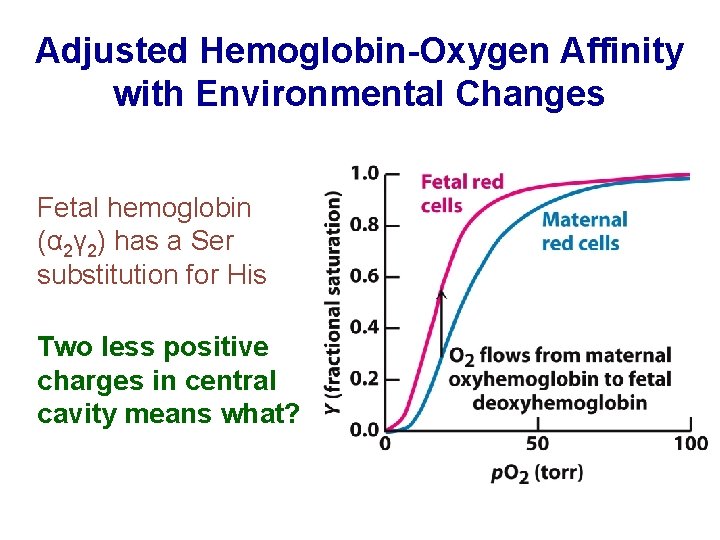
Adjusted Hemoglobin-Oxygen Affinity with Environmental Changes Fetal hemoglobin (α 2γ 2) has a Ser substitution for His Two less positive charges in central cavity means what?
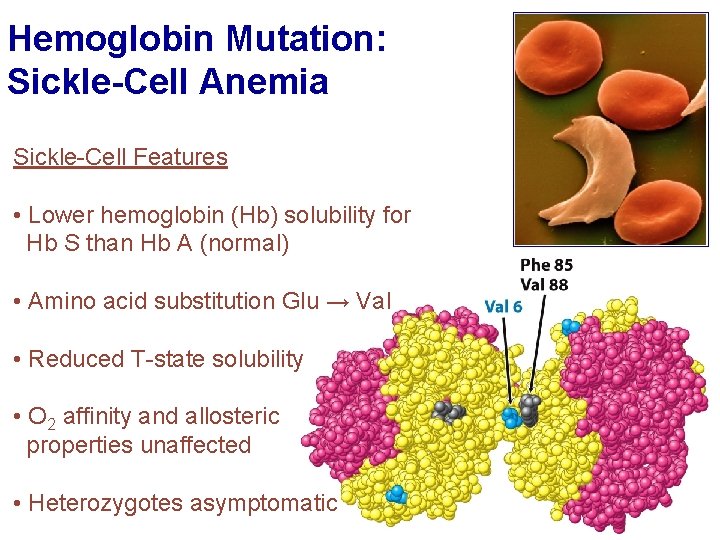
Hemoglobin Mutation: Sickle-Cell Anemia Sickle-Cell Features • Lower hemoglobin (Hb) solubility for Hb S than Hb A (normal) • Amino acid substitution Glu → Val • Reduced T-state solubility • O 2 affinity and allosteric properties unaffected • Heterozygotes asymptomatic
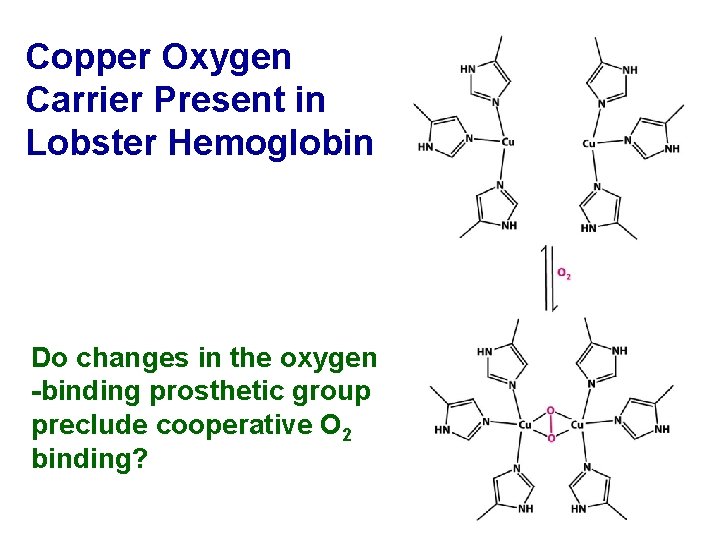
Copper Oxygen Carrier Present in Lobster Hemoglobin Do changes in the oxygen -binding prosthetic group preclude cooperative O 2 binding?
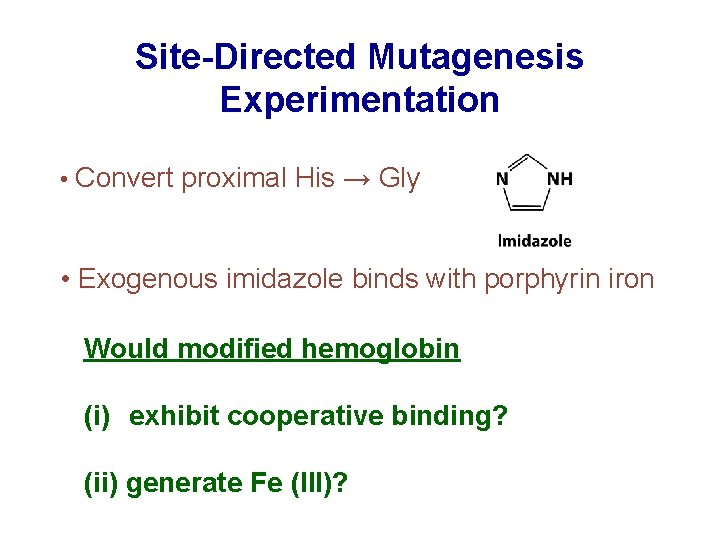
Site-Directed Mutagenesis Experimentation • Convert proximal His → Gly • Exogenous imidazole binds with porphyrin iron Would modified hemoglobin (i) exhibit cooperative binding? (ii) generate Fe (III)?
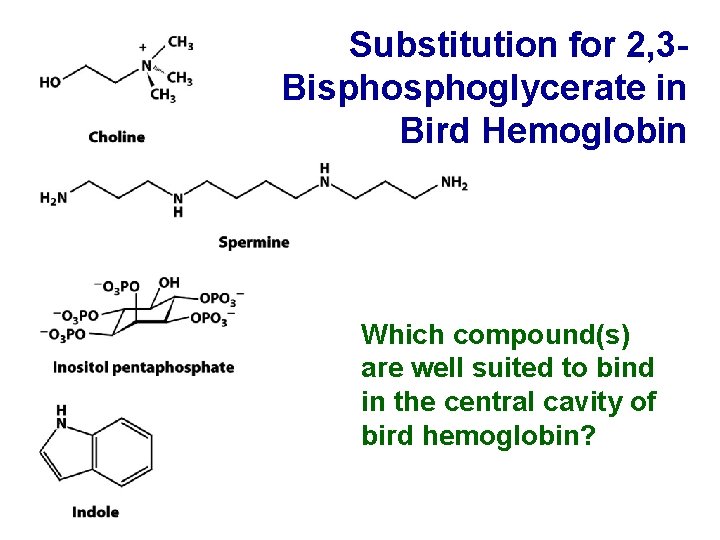
Substitution for 2, 3 Bisphoglycerate in Bird Hemoglobin Which compound(s) are well suited to bind in the central cavity of bird hemoglobin?
![Hemoglobin Fractional Saturation Curve Shift Curve matching: Increase in [CO 2] Increase in 2, Hemoglobin Fractional Saturation Curve Shift Curve matching: Increase in [CO 2] Increase in 2,](http://slidetodoc.com/presentation_image_h2/3474c7e02307c8d034b27e6f36b71c47/image-20.jpg)
Hemoglobin Fractional Saturation Curve Shift Curve matching: Increase in [CO 2] Increase in 2, 3 -BPG Increase in p. H Loss of 4° structure Physiological [CO 2] and [2, 3 -BPG] at p. H = 7
- Slides: 20