OxidationReduction Reactions LEO SAYS GER Oxidation Numbers The

“Oxidation-Reduction Reactions” LEO SAYS GER

Oxidation Numbers The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred. • An “oxidation number” is a positive or negative number assigned to an atom
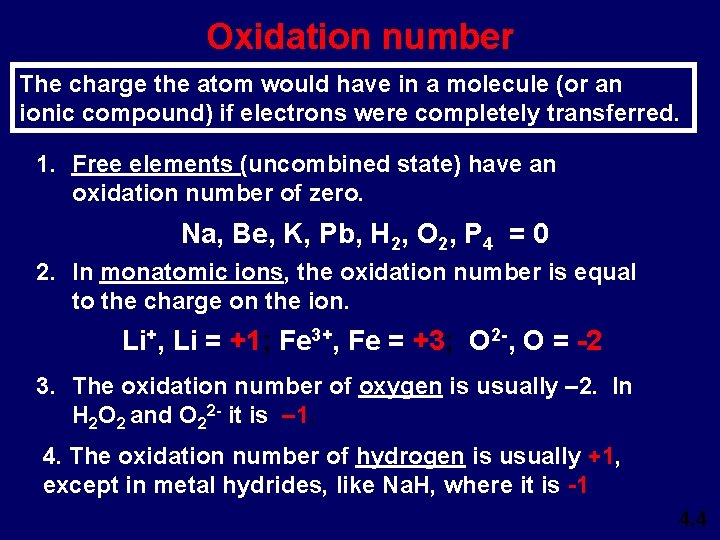
Oxidation number The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred. 1. Free elements (uncombined state) have an oxidation number of zero. Na, Be, K, Pb, H 2, O 2, P 4 = 0 2. In monatomic ions, the oxidation number is equal to the charge on the ion. Li+, Li = +1; Fe 3+, Fe = +3; O 2 -, O = -2 3. The oxidation number of oxygen is usually – 2. In H 2 O 2 and O 22 - it is – 1. 4. The oxidation number of hydrogen is usually +1, except in metal hydrides, like Na. H, where it is -1 4. 4

Oxidation Numbers 5. Group 1 metals have an oxidation number of +1 K+1, Li+1, Na+1 6. Group 2 metals have an oxidation number of +2 Ca+2, Mg+2
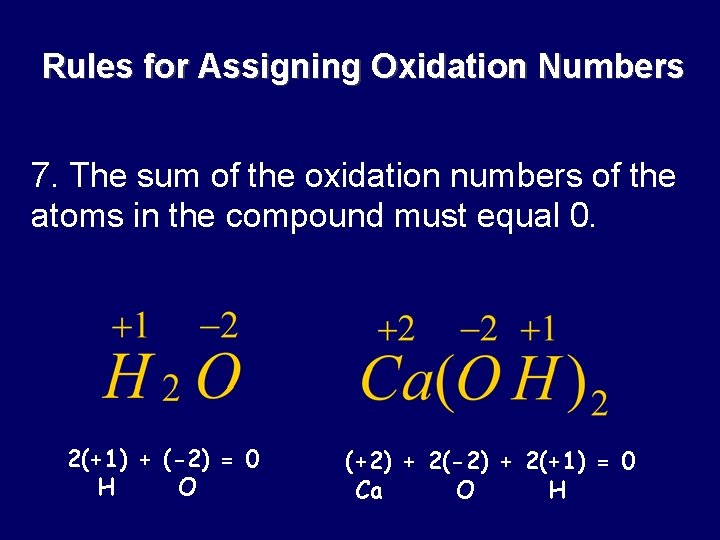
Rules for Assigning Oxidation Numbers 7. The sum of the oxidation numbers of the atoms in the compound must equal 0. 2(+1) + (-2) = 0 H O (+2) + 2(-2) + 2(+1) = 0 Ca O H
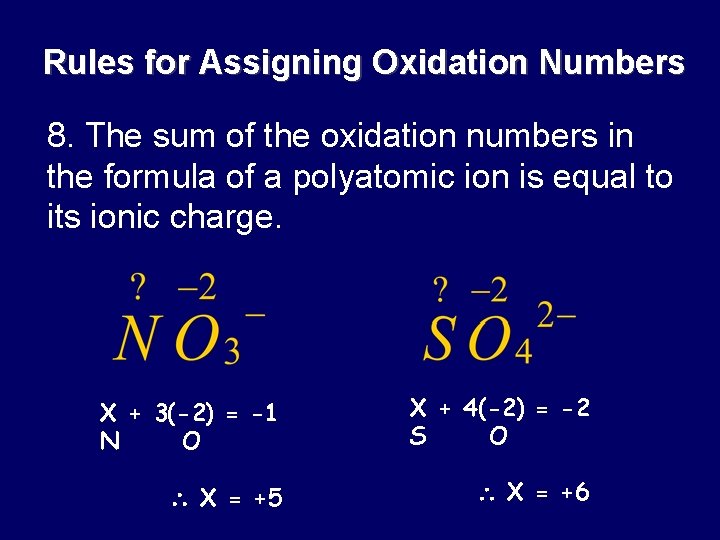
Rules for Assigning Oxidation Numbers 8. The sum of the oxidation numbers in the formula of a polyatomic ion is equal to its ionic charge. X + 3(-2) = -1 N O X + 4(-2) = -2 S O X = +5 X = +6
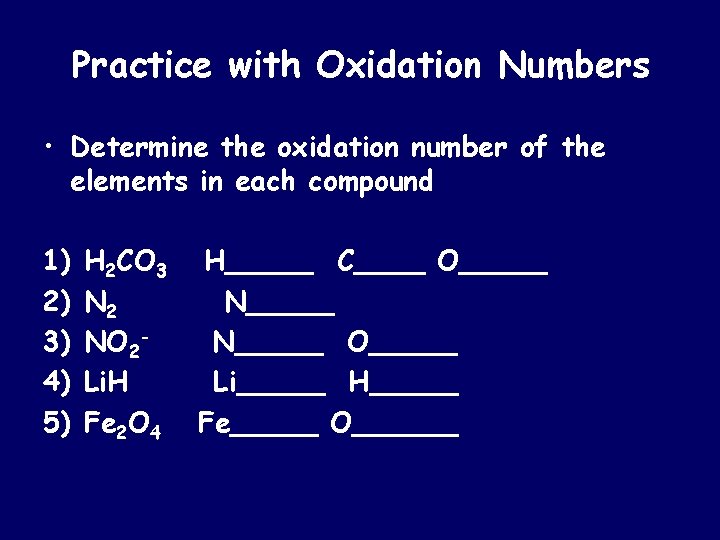
Practice with Oxidation Numbers • Determine the oxidation number of the elements in each compound 1) 2) 3) 4) 5) H 2 CO 3 N 2 NO 2 Li. H Fe 2 O 4 H_____ C____ O_____ N_____ O_____ Li_____ H_____ Fe_____ O______

Oxidation and Reduction (Redox) Reactions Oxidation and reduction always occur simultaneously The substance losing electrons is oxidized, while the substance gaining electrons is reduced.
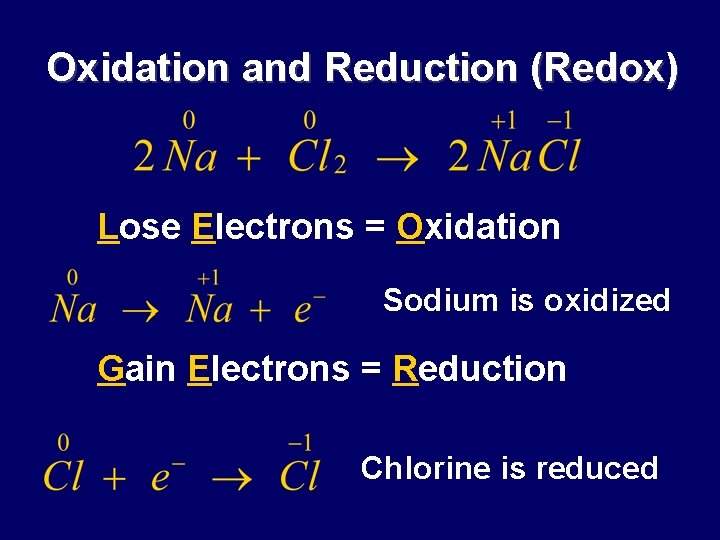
Oxidation and Reduction (Redox) Lose Electrons = Oxidation Sodium is oxidized Gain Electrons = Reduction Chlorine is reduced

LEO says GER : Cr+ + Sn 4+ → Cr 3+ + Sn 2+ Cr+: loses 2 electrons (oxidized), Sn 4+: gains 2 electrons (reduced)

Identifying Redox Reactions 1. Assign oxidation numbers to all atoms in the equation 2. Compare oxidation numbers from the reactant side to the product side. – If a redox reaction has occurred, you will find that TWO numbers have changed 3. The element oxidized is the one whose oxidation number increased 4. The element reduced is the one whose oxidation number decreased.
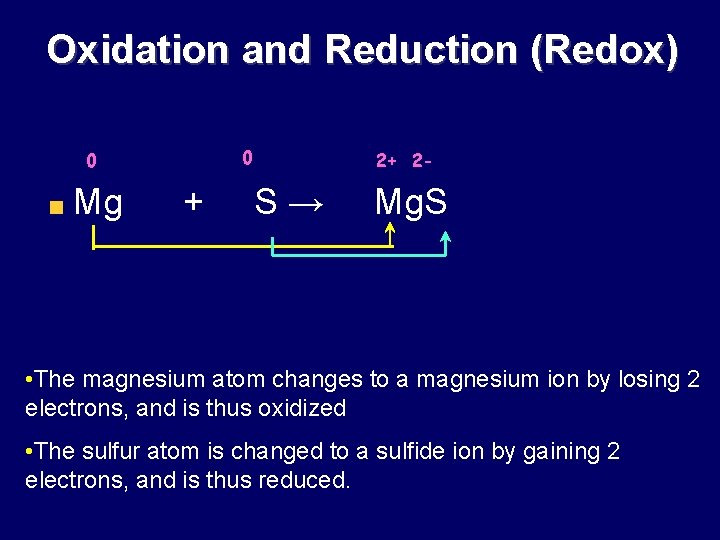
Oxidation and Reduction (Redox) 0 0 Mg + 2+ 2 - S→ Mg. S • The magnesium atom changes to a magnesium ion by losing 2 electrons, and is thus oxidized • The sulfur atom is changed to a sulfide ion by gaining 2 electrons, and is thus reduced.

Not All Reactions are Redox Reactions - Reactions in which there has been no change in oxidation number are not redox reactions. Examples:

Identifying Redox Equations In an electrical storm, oxygen and nitrogen react to form nitrogen monoxide: N 2(g) + O 2(g) → 2 NO(g) YES! • Is this a redox reaction?
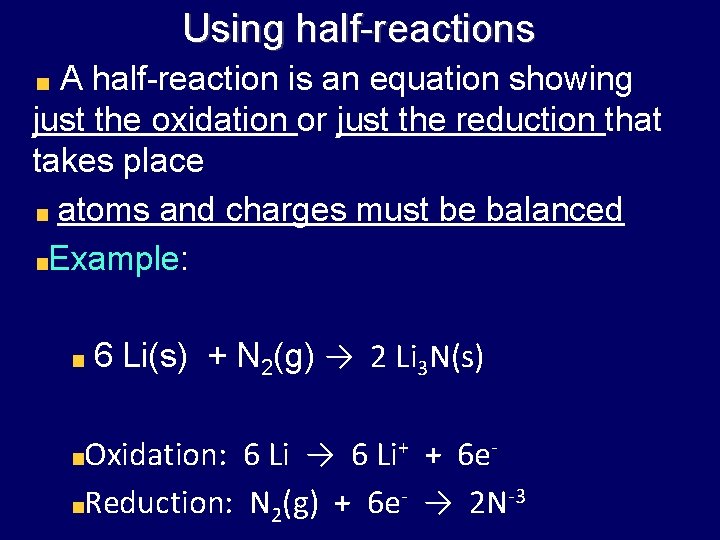
Using half-reactions A half-reaction is an equation showing just the oxidation or just the reduction that takes place atoms and charges must be balanced Example: 6 Li(s) + N 2(g) → 2 Li 3 N(s) Oxidation: 6 Li → 6 Li+ + 6 e. Reduction: N 2(g) + 6 e- → 2 N-3

• Try One: • 2 K(s) + Br 2(g) → 2 KBr (s) • Oxidation: • Reduction:
- Slides: 16