OxidationReduction Reactions Identifying oxidation agents oxidant reducing agent


















- Slides: 22

Oxidation-Reduction Reactions Identifying oxidation agents, oxidant, reducing agent, reductant, what is oxidized, what is reduced.
Do you like Lions or Petroleum? Le. O Ge. R vs. Oi. L Ri. G

Do you like Lions or Petroleum? Le. O Ge. R Losing electrons Oxidation Gaining electrons Reduced vs. Oi. L Ri. G Oxidation is Losing electrons Reduction is Gaining electrons

Make sure you get it straight in your head! Oxidation is the loss of electrons, or the increase in oxidation state (number). Reduction is the gain of electrons, or the decrease in oxidation state (number).

History Oxidation originally referred to the reaction with oxygen (the first studied oxidizing agent) to form oxides. Later was expanded to reference loss of electrons.

History Reduction first referred to the loss of weight of heating a metal ore (metal oxide) to extract the metal. Lavoisier showed that this was due to the loss of oxygen. Later it was realized that the metal atoms gained electrons. Eventually expanded to generally refer to gaining of electrons.

Vocabulary When something is oxidized, it is the reducing agent. That means that it causes something else to be reduced while it is oxidized. It can also be referred to as a reducer or reductant. It also can referred to as being reductive or reducing.

Vocabulary When something is reduced, it is the oxidizing agent. That means that it causes something else to be oxidized while it is reduced. It can also be referred to as a oxidizer or oxidant. It also can referred to as being oxidative or oxidizing.

LEORA says GEROA Losing Electrons Oxidation Reducing Agent Gaining Electrons Reduced Oxidizing Agent

Practice First determine the oxidation states of the elements, then determine which is oxidized and which is reduced. Lastly, identify the oxidizing agent and the reducing agent.

Guideline #1: Elemental atoms Atoms in their elemental form always have an oxidation number of ZERO! Ex. H 2 : each H atom has an oxidation number of 0. (H 0) P 4 : each P atom has an oxidation number of 0. (P 0) Cu : Cu has an oxidation number of 0. (Cu 0)

Guideline #2: Monatomic ions have the same oxidation number as the ionic charge. Ex. S-2 : each S atom has an oxidation number of -2. (S 2 -) P-3 : each P atom has an oxidation number of -3. (P 3 -) Al : Al has an oxidation number of +3. (Al 3+)

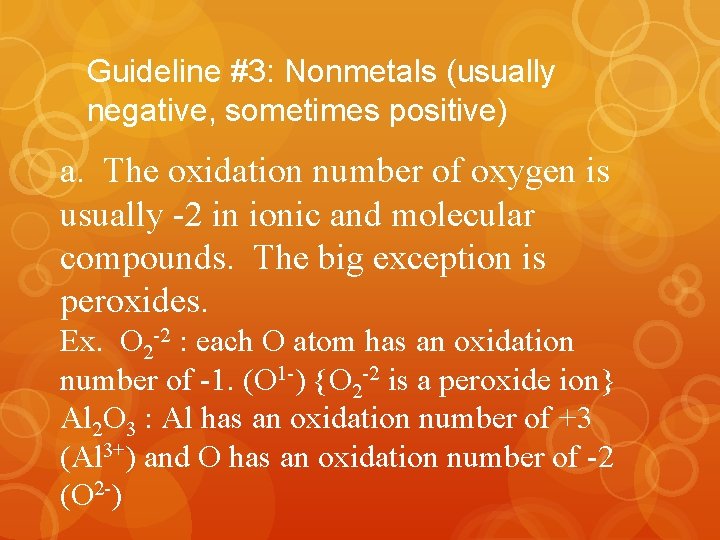
Guideline #3: Nonmetals (usually negative, sometimes positive) a. The oxidation number of oxygen is usually -2 in ionic and molecular compounds. The big exception is peroxides. Ex. O 2 -2 : each O atom has an oxidation number of -1. (O 1 -) {O 2 -2 is a peroxide ion} Al 2 O 3 : Al has an oxidation number of +3 (Al 3+) and O has an oxidation number of -2 (O 2 -)

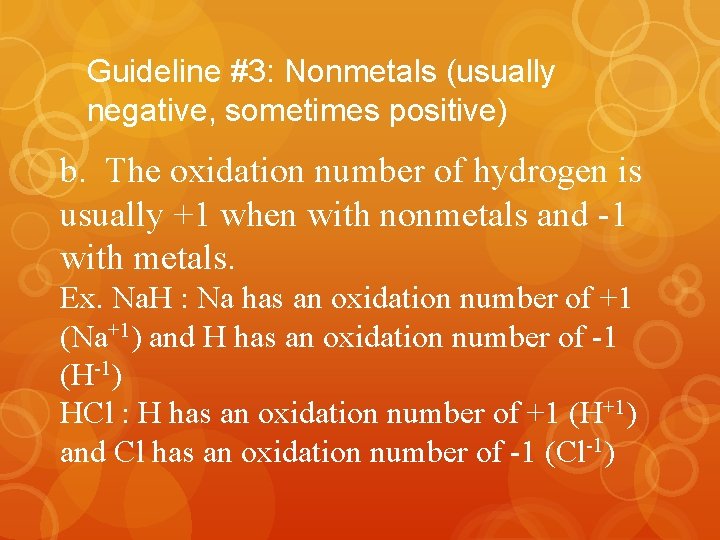
Guideline #3: Nonmetals (usually negative, sometimes positive) b. The oxidation number of hydrogen is usually +1 when with nonmetals and -1 with metals. Ex. Na. H : Na has an oxidation number of +1 (Na+1) and H has an oxidation number of -1 (H-1) HCl : H has an oxidation number of +1 (H+1) and Cl has an oxidation number of -1 (Cl-1)

Guideline #3: Nonmetals (usually negative, sometimes positive) c. The oxidation number of fluorine is always -1. Other halogens have negative oxidation numbers unless with oxygen, then it is positive. Ex. OCl- : Cl has an oxidation number of +1 (Cl+1) and O has an oxidation number of -2 (O-2) HF : H has an oxidation number of +1 (H+1) and F has an oxidation number of -1 (F-1)

Guideline #4: Sum of oxidation numbers The sum of the oxidation numbers of a neutral compound is ZERO! The sum of the oxidation numbers of a polyatomic ion is the charge of the ion. Ex. H 3 O+ : H has an oxidation number of +1 (H+1) and O has an oxidation number of -2 (O -2). Total is +1 K 2 O : K has an oxidation number of +1 (K+1) and O has an oxidation number of -2 (O-2)

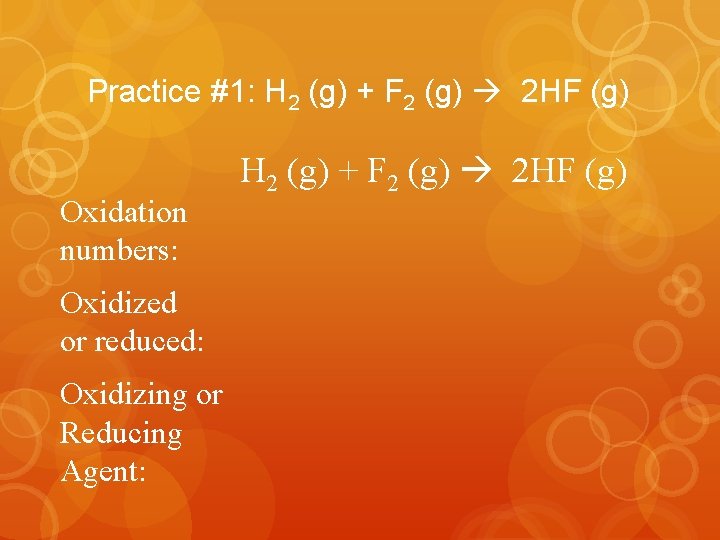
Practice #1: H 2 (g) + F 2 (g) 2 HF (g) Oxidation numbers: Oxidized or reduced: Oxidizing or Reducing Agent: H 2 (g) + F 2 (g) 2 HF (g)

Practice #1: H 2 (g) + F 2 (g) HF (g) Oxidation numbers: H 2 (g) + F 2 (g) HF (g) 0 0 +1 -1 Oxidized or Reduced: oxidized reduced Oxidizing or Reducing Agent: Reducing A Oxidizing A

Practice #2: Fe (s) + Cu. SO 4 (aq) Cu (s) + Fe 2(SO 4)3 (aq) Oxidation numbers: Oxidized or Reduced: Oxidizing or Reducing Agent:

Practice #2: Fe (s) + Cu. SO 4 (aq) Cu (s) + Fe 2(SO 4)3 (aq) O#: 0 +2 +6 -8 O or R: Fe: oxidized 0 +3 +6 -2 Cu: reduced OA or RA: Fe: Reducing agent Cu: Oxidizing agent

Practice #3: Mn+2 (aq) + Na. Bi. O 3 (s) Bi+3 (aq) + Mn. O 4 -1 (aq) Oxidation Number: Oxidation or Reduction: Oxidizing Agent or Reducing Agent:

Practice #3: Mn+2 (aq) + Na. Bi. O 3 (s) Bi+3 (aq) + Mn. O 4 -1 (aq) O#: +2 +1 +5 -6 O or R: Mn: oxidized +3 +7 -8 Bi: reduced OA or RA: Mn: Reducing agent Bi: Oxidizing agent