Oxidation states of vanadium AH Chemistry Unit 1c










- Slides: 14

Oxidation states of vanadium AH Chemistry, Unit 1(c)

Aims • Illustrate the presence of several oxidation states for vanadium • Show it is possible to change from one oxidation state to another

What are “oxidation states”? • The actual charge of an atom if the atom is a monatomic ion, or the hypothetical charge assigned to an atom by certain rules

Rules 1. Elements are 0 2. Atoms of monatomic ions have charge of ion 3. O is -2 in most compounds 4. H is +1 in most compounds 5. F is -1 in all compounds 6. Sum of numbers in a compound is 0; in a polyatomic ion is charge of ion

Example • Oxidation number of Cl in: – HCl. O 4 (perchloric acid) – Cl. O 3 - (chlorate ion)

The significance of transition metals • Transition metals exhibit variable oxidation states of differing stability • A change in oxidation state can bring about a change in colour

Oxidation and reduction • Oxidation can be considered as an increase in oxidation number • Reduction can be considered as a decrease in oxidation number • Compounds with metals in a high oxidation state tend to be oxidising agents whereas compounds with metals in a low oxidation state are often reducing agents

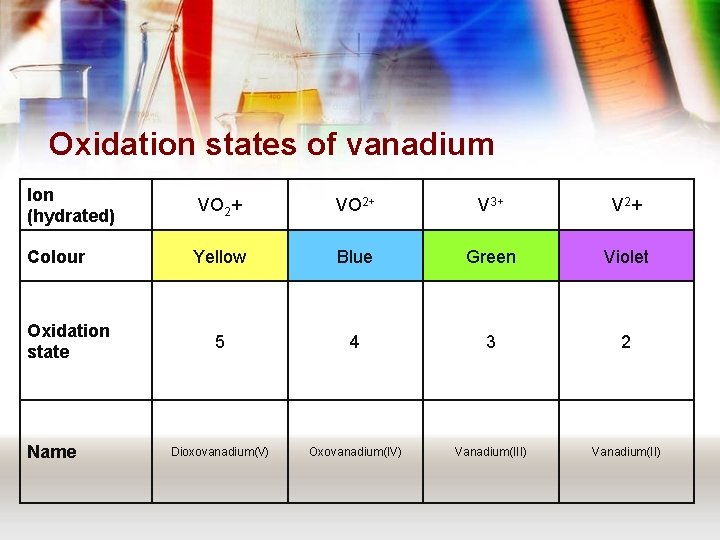
Oxidation states of vanadium Ion (hydrated) VO 2+ V 3+ V 2+ Colour Yellow Blue Green Violet 5 4 3 2 Dioxovanadium(V) Oxovanadium(IV) Vanadium(III) Vanadium(II) Oxidation state Name

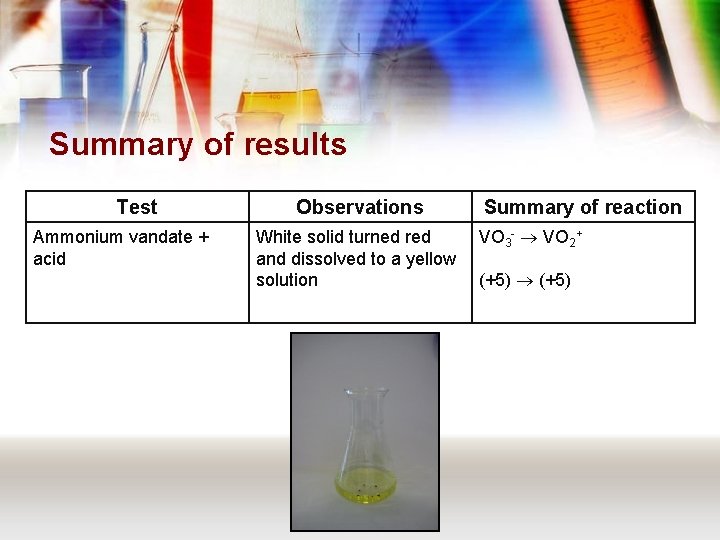
Summary of results Test Ammonium vandate + acid Observations White solid turned red and dissolved to a yellow solution Summary of reaction VO 3 - VO 2+ (+5)

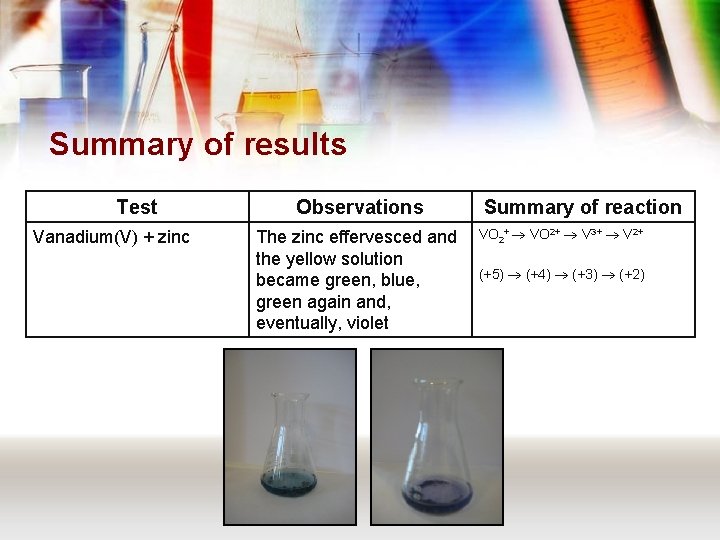
Summary of results Test Vanadium(V) + zinc Observations The zinc effervesced and the yellow solution became green, blue, green again and, eventually, violet Summary of reaction VO 2+ V 3+ V 2+ (+5) (+4) (+3) (+2)

Summary of results Test Vanadium(II) + manganate(VII) Observations The violet solution became green, blue, green-yellow and, finally, pink Summary of reaction V 2+ V 3+ VO 2+ (+2) (+3) (+4) (+5)

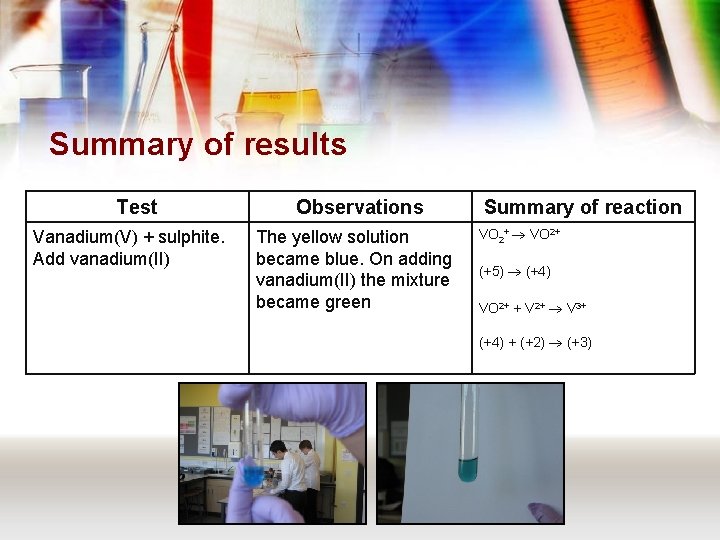
Summary of results Test Vanadium(V) + sulphite. Add vanadium(II) Observations The yellow solution became blue. On adding vanadium(II) the mixture became green Summary of reaction VO 2+ (+5) (+4) VO 2+ + V 2+ V 3+ (+4) + (+2) (+3)

Summary of results Test Vanadium(V) + iodide + thiosulphate Observations The yellow solution became a muddy brown. Addition of thiosulphate gave a clear blue solution Summary of reaction VO 2+ (+5) (+4)
