Oxidation Reduction Revision AG Definitions Oxidation is addition





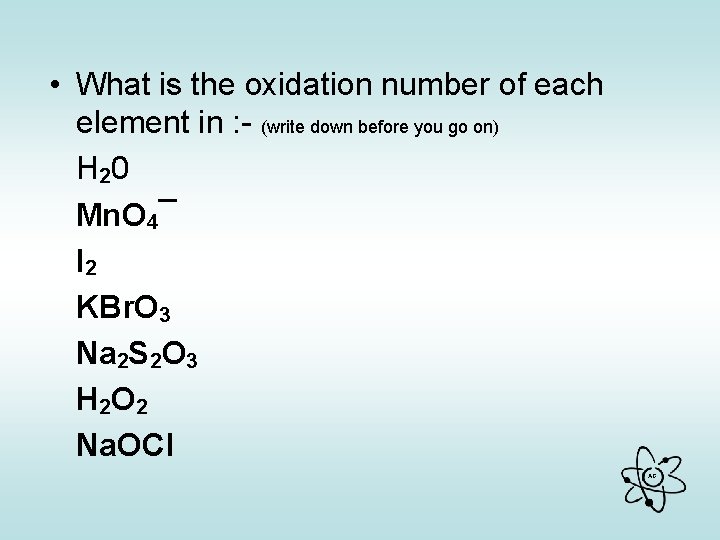




- Slides: 26

Oxidation Reduction Revision AG

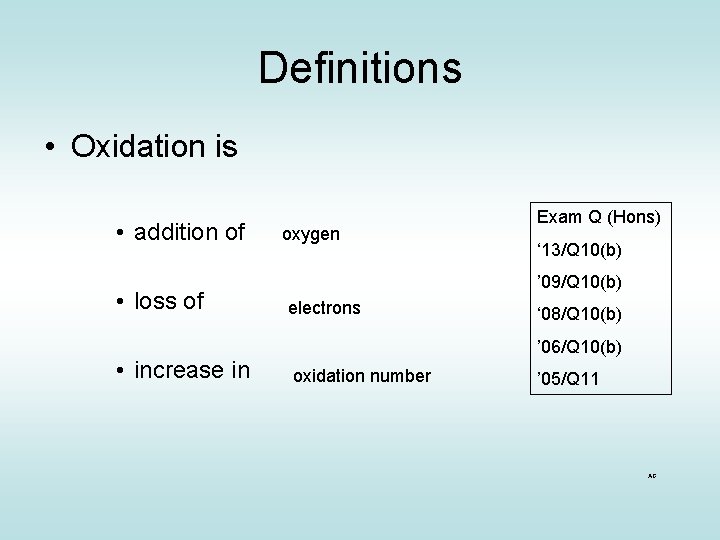
Definitions • Oxidation is • addition of • loss of • increase in oxygen Exam Q (Hons) ‘ 13/Q 10(b) ’ 09/Q 10(b) electrons ‘ 08/Q 10(b) ’ 06/Q 10(b) oxidation number ’ 05/Q 11 AG

• Reduction is • loss of oxygen • gain of electrons • decrease in oxidation number AG

More… • An oxidising agent causes(allows) oxidation and is itself reduced. • A reducing agent causes(allows) reduction and is itself oxidised. • What is a redox reaction? AG

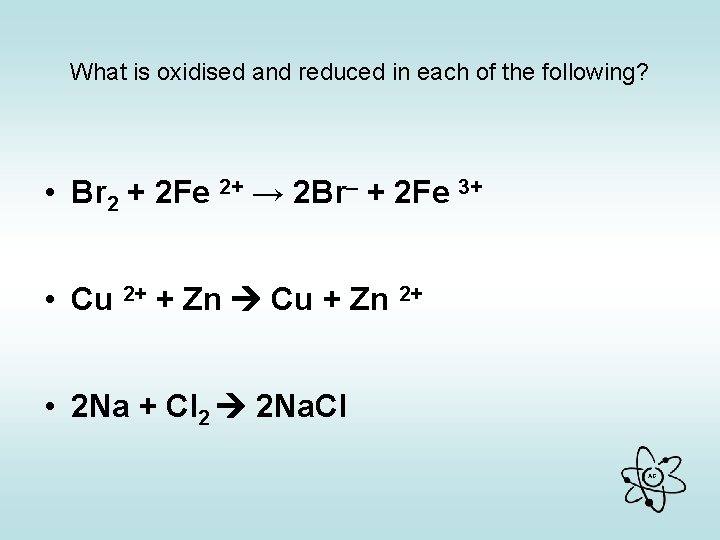
What is oxidised and reduced in each of the following? • Br 2 + 2 Fe 2+ → 2 Br– + 2 Fe 3+ • Cu 2+ + Zn Cu + Zn 2+ • 2 Na + Cl 2 2 Na. Cl AG

Learning Check Can I give definition for a) Oxidation In terms of • Electron transfer • Oxygen transfer • Oxidation number Repeat for reduction NOT The End click to go on AG

Oxidation Number • Oxidation number is defined as Ø The charge an atom has Ø Or appears to have Ø When electrons are distributed Ø according to certain rules AG

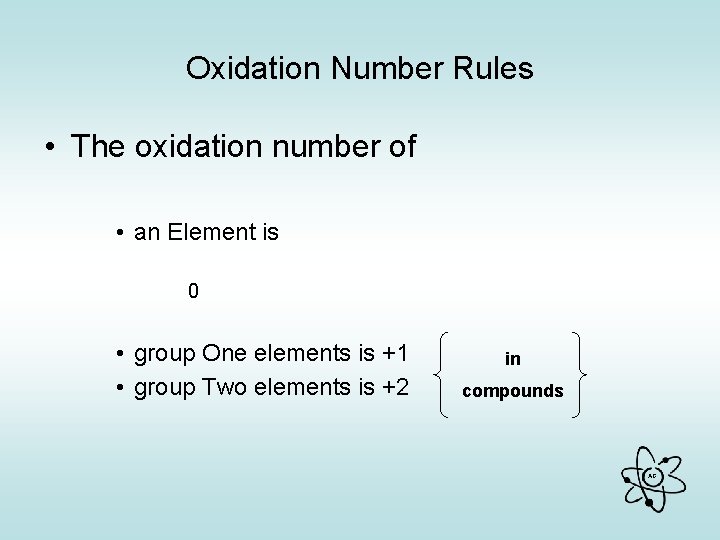
Oxidation Number Rules • The oxidation number of • an Element is 0 • group One elements is +1 • group Two elements is +2 in compounds AG

The oxidation number of an ion is equal to the charge on the ion • halogens is -1 (in binary compounds) (except ……? ? ) AG

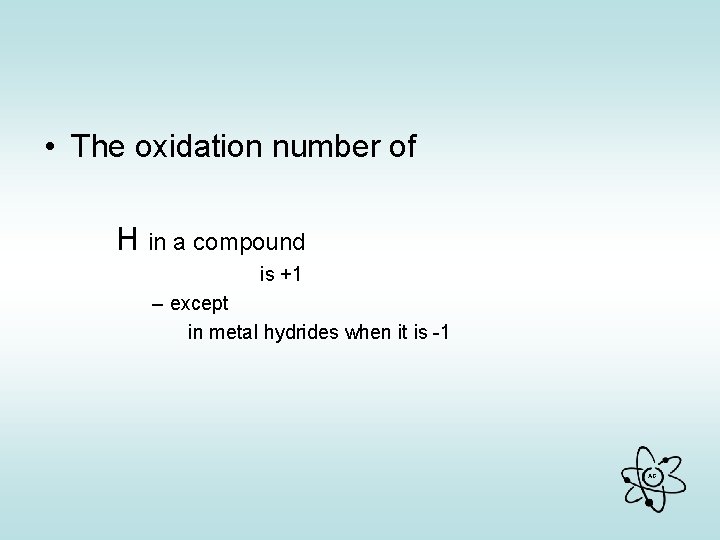
• The oxidation number of H in a compound is +1 – except in metal hydrides when it is -1 AG

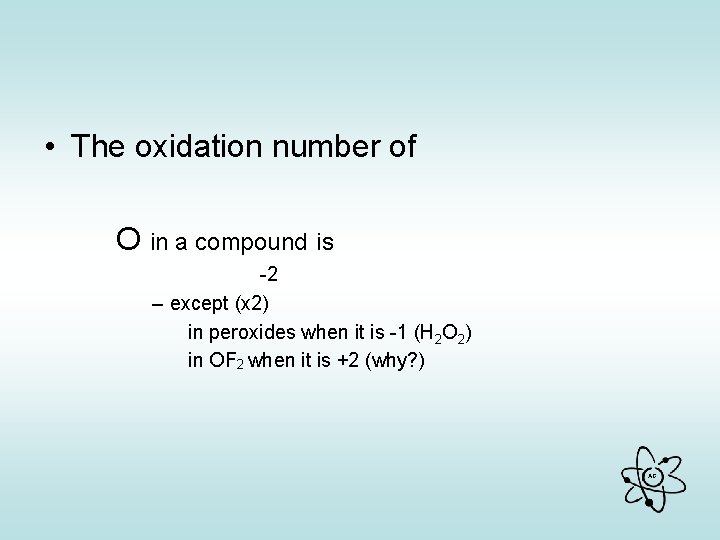
• The oxidation number of O in a compound is -2 – except (x 2) in peroxides when it is -1 (H 2 O 2) in OF 2 when it is +2 (why? ) AG

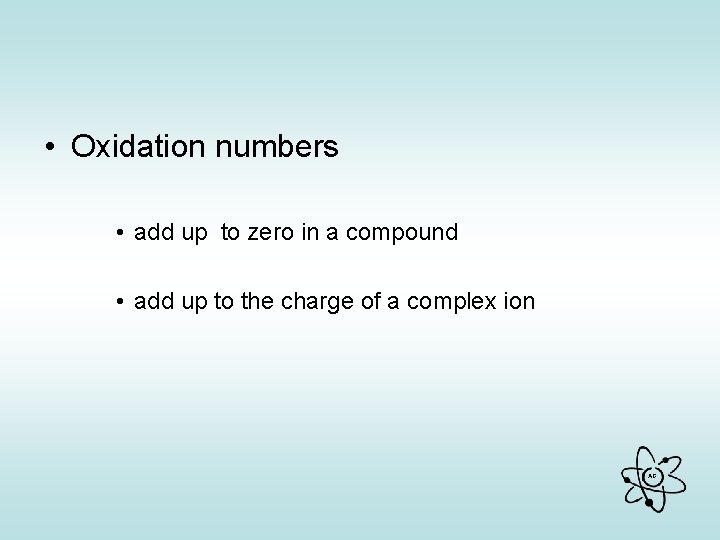
• Oxidation numbers • add up to zero in a compound • add up to the charge of a complex ion AG
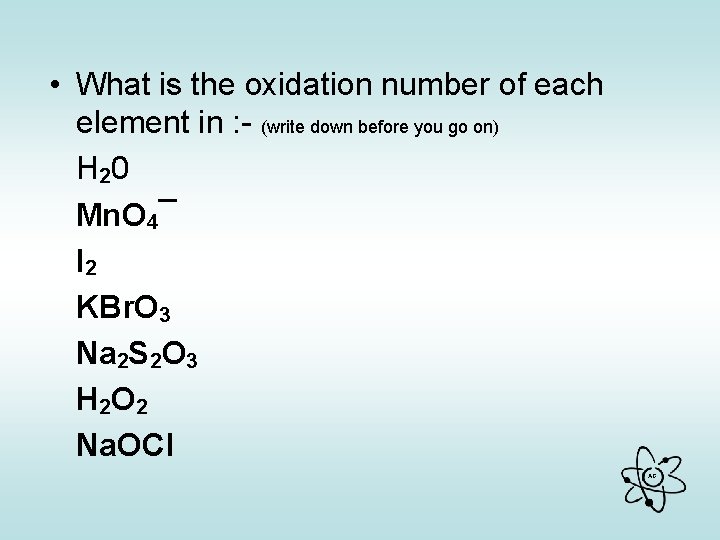
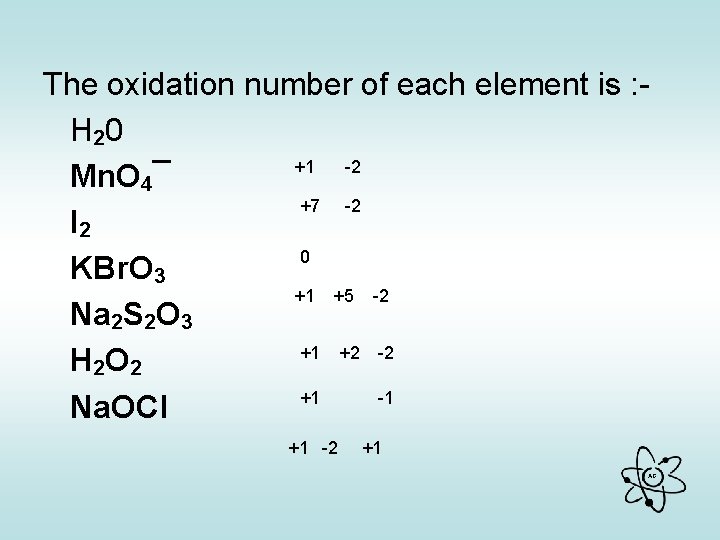
• What is the oxidation number of each element in : - (write down before you go on) H 20 Mn. O 4¯ I 2 KBr. O 3 Na 2 S 2 O 3 H 2 O 2 Na. OCl AG

The oxidation number of each element is : H 20 +1 -2 Mn. O 4¯ +7 -2 I 2 0 KBr. O 3 +1 +5 -2 Na 2 S 2 O 3 +1 +2 -2 H 2 O 2 +1 -1 Na. OCl +1 -2 +1 AG

Learning Check Can I give the oxidation number RULE for a) Oxygen b) Hydrogen c) free element d) Neutral atom (sum) e) Ion (simple and complex) f) Group 1 element g) Group 2 element h) HALOGEN STILL NOT The End - click to go on AG

Balancing Equations with oxidation numbers STEPS 1. Assign oxidation numbers 2. Identify what is oxidised and reduced 3. Write half equation SIDE by SIDE for each (showing number of electrons on the move for one atom of each) 4. 5. 6. 7. 8. Rewrite for the number of atoms given e. g. Cr 2 Balance the electrons REWRITE the original equation using these “prefixes” Balance remainder by inspection CHECK – do the charges on each side cancel out? ? AG

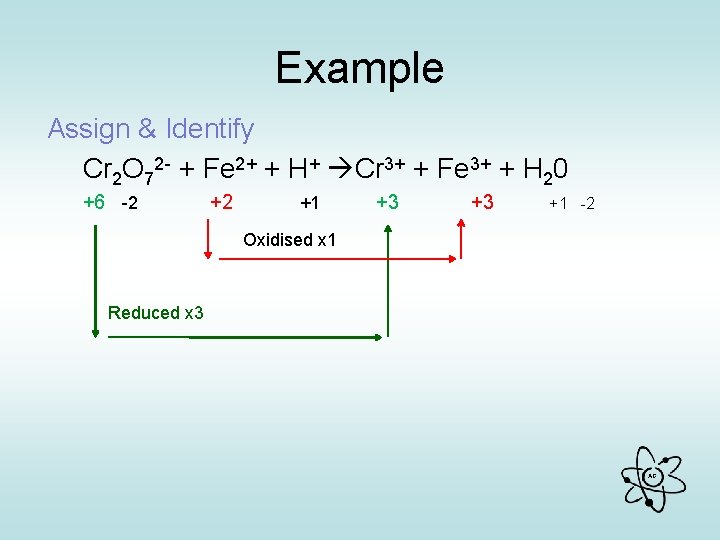
Example Assign & Identify Cr 2 O 72 - + Fe 2+ + H+ Cr 3+ + Fe 3+ + H 20 +6 -2 +2 +1 +3 +3 +1 -2 Oxidised x 1 Reduced x 3 AG

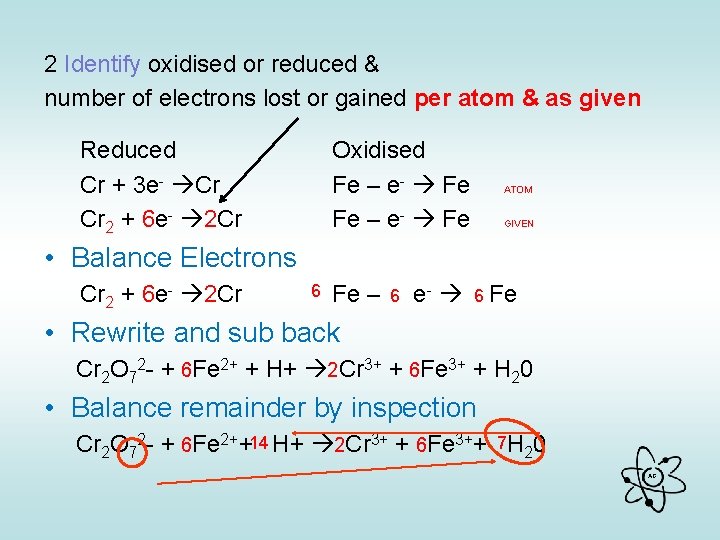
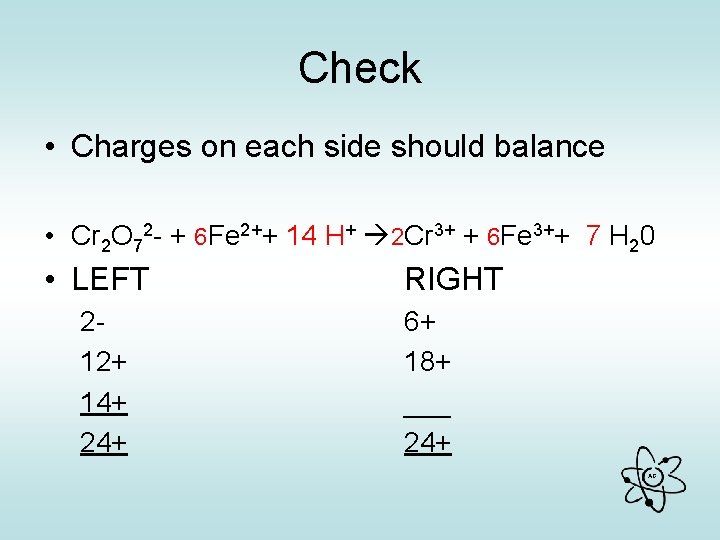
2 Identify oxidised or reduced & number of electrons lost or gained per atom & as given Reduced Cr + 3 e- Cr Cr 2 + 6 e- 2 Cr Oxidised Fe – e- Fe ATOM GIVEN • Balance Electrons Cr 2 + 6 e- 2 Cr 6 Fe – 6 e- 6 Fe • Rewrite and sub back Cr 2 O 72 - + 6 Fe 2+ + H+ 2 Cr 3+ + 6 Fe 3+ + H 20 • Balance remainder by inspection Cr 2 O 72 - + 6 Fe 2++14 H+ 2 Cr 3+ + 6 Fe 3++ 7 H 20 AG

Check • Charges on each side should balance • Cr 2 O 72 - + 6 Fe 2++ 14 H+ 2 Cr 3+ + 6 Fe 3++ 7 H 20 • LEFT 212+ 14+ 24+ RIGHT 6+ 18+ ___ 24+ AG

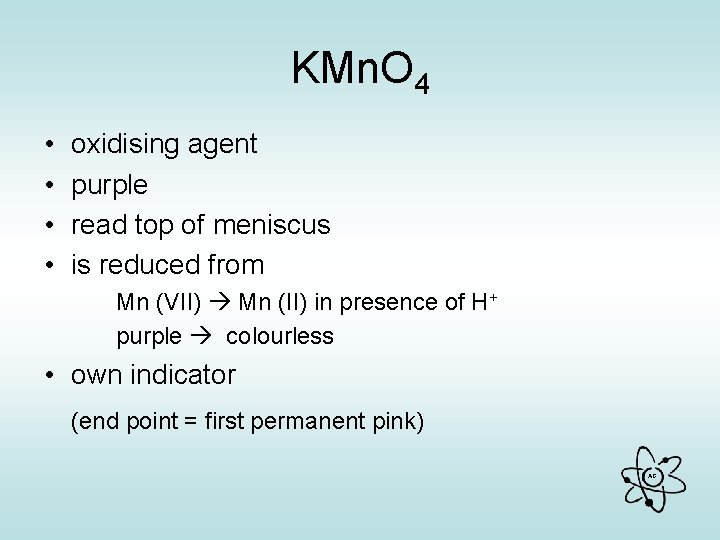
KMn. O 4 • • oxidising agent purple read top of meniscus is reduced from Mn (VII) Mn (II) in presence of H+ purple colourless • own indicator (end point = first permanent pink) AG

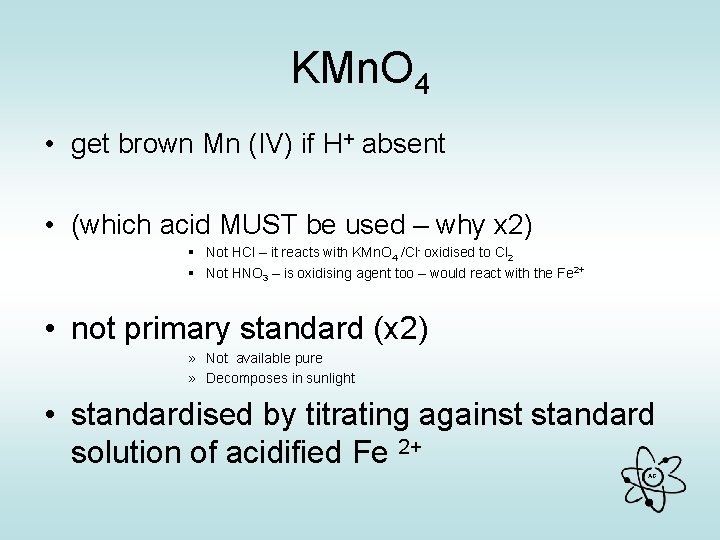
KMn. O 4 • get brown Mn (IV) if H+ absent • (which acid MUST be used – why x 2) § Not HCl – it reacts with KMn. O 4 /Cl- oxidised to Cl 2 § Not HNO 3 – is oxidising agent too – would react with the Fe 2+ • not primary standard (x 2) » Not available pure » Decomposes in sunlight • standardised by titrating against standard solution of acidified Fe 2+ AG

H 2 SO 4 • added during KMn. O 4 titrations to provide H+ and ensure the complete reduction of Mn (VII) Mn (II) and prevent formation of Mn (IV) (brown) • added during prep. of Fe (II) solutions to prevent oxidation of Fe 2+ to Fe 3+ by oxygen in the air ( why does this matter? ) AG

Na 2 S 2 O 3 • S 2 O 3 2 - ion • reducing agent • used in photography • not primary standard – why ? • standardised by titrating against I 2 • starch indicator – when added and why • colour change at end point ? AG

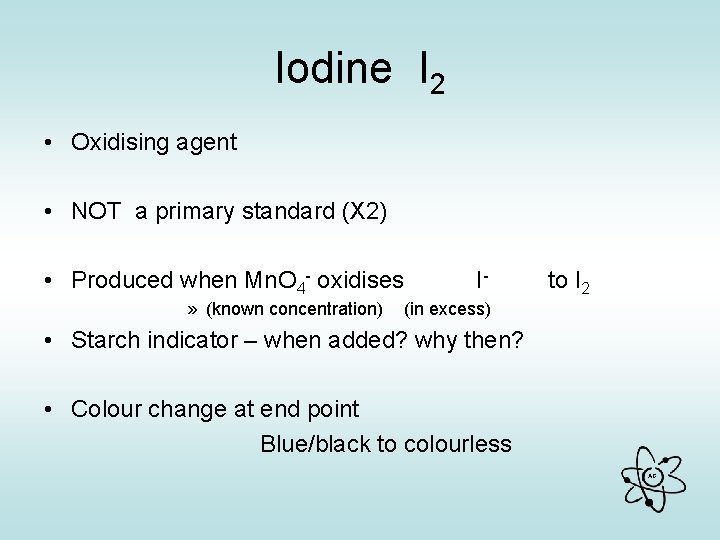
Iodine I 2 • Oxidising agent • NOT a primary standard (X 2) • Produced when Mn. O 4 - oxidises » (known concentration) I- to I 2 (in excess) • Starch indicator – when added? why then? • Colour change at end point Blue/black to colourless AG

Bleach • sodium hypochlorite Na+Cl. O • bleach diluted x 10 with distilled water not de-ionised water (why? ) NB • Cl. O- oxidises I- to I 2 • I 2 v. thiosulfate remember dilution factor in calculations • starch indicator as before AG

Learning Check Can I • name 2 oxidising agents • Name 2 reducing agents • say why KMn. O 4 isn’t primary standard • say why Na 2 S 2 O 3 isn’t primary standard • say why I 2 isn’t primary standard • say why bleach isn’t primary standard • Give details of titrations + indicators for each FINALLY The End AG