Oxidation Reduction redox Oxidation and reduction occur simultaneously



- Slides: 7

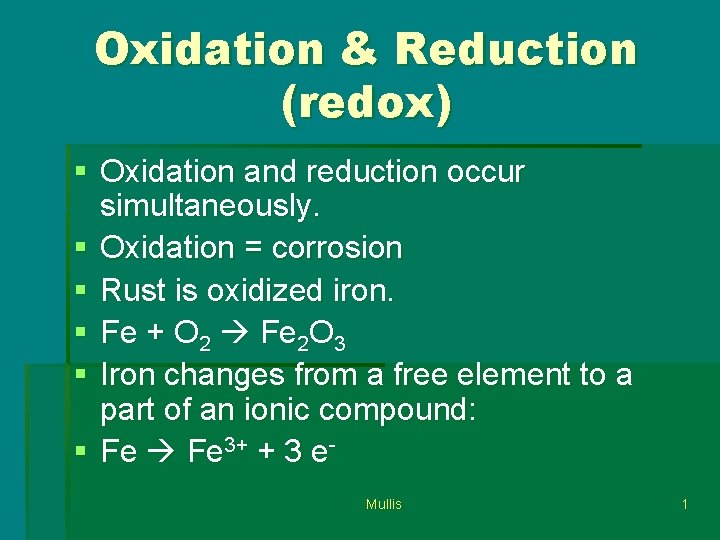
Oxidation & Reduction (redox) § Oxidation and reduction occur simultaneously. § Oxidation = corrosion § Rust is oxidized iron. § Fe + O 2 Fe 2 O 3 § Iron changes from a free element to a part of an ionic compound: § Fe 3+ + 3 e. Mullis 1

Oxidation § When a substance loses electrons, it undergoes oxidation. Ca(s) + 2 H+(aq) Ca 2+(aq) + H 2(g) § Ca lost 2 electrons to the H+ ions. § The metal Ca became an ion: Ca 2+ § Ca has been oxidized. § H+ was the oxidizing agent. (It made oxidation happen. ) Mullis 2

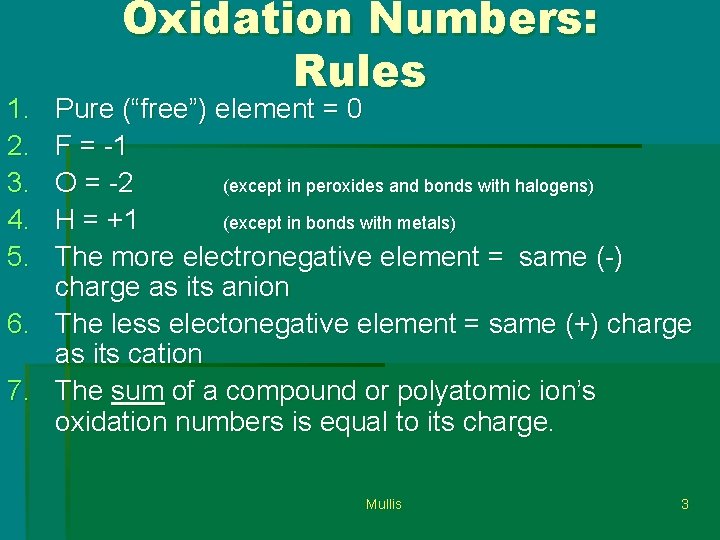
1. 2. 3. 4. 5. Oxidation Numbers: Rules Pure (“free”) element = 0 F = -1 O = -2 (except in peroxides and bonds with halogens) H = +1 (except in bonds with metals) The more electronegative element = same (-) charge as its anion 6. The less electonegative element = same (+) charge as its cation 7. The sum of a compound or polyatomic ion’s oxidation numbers is equal to its charge. Mullis 3

LEO says GER (Identifying ½ Reactions) § Lose Electrons Oxidation / Gain Electrons Reduction § Assign oxidation numbers 1 st § Ex. H 2 + Cl 2 2 H Cl 0 0 +1 -1 § Pick the element that goes down in Oxidation Number: Here, this is Cl (0 -1). Its half reaction is: § Cl 2 + 2 e- 2 Cl. Cl is reduced § Pick the element that goes up in Oxidation Number: Here, this is H (0 +1). Its half reaction is: § H 2 2 H+ + 2 e. H is oxidized Mullis 4

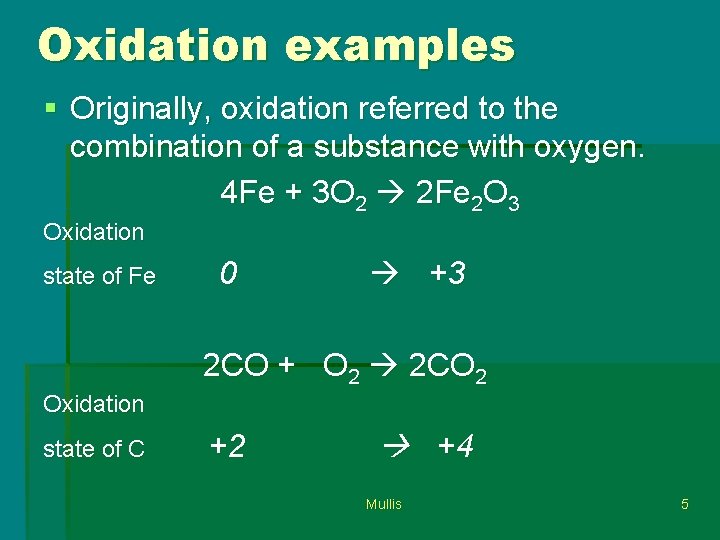
Oxidation examples § Originally, oxidation referred to the combination of a substance with oxygen. 4 Fe + 3 O 2 2 Fe 2 O 3 Oxidation state of Fe 0 +3 2 CO + O 2 2 CO 2 Oxidation state of C +2 +4 Mullis 5

Reduction § When a substance loses electrons, it undergoes oxidation. 2 Ca(s) + O 2(g) 2 Ca. O (s) § O gained 2 electrons to become O 2 - in Ca. O. § The neutral O 2 became an ion: O 2§ O 2 has been reduced. § In all reduction-oxidation reactions, one species is reduced at the same time as another is oxidized. Mullis 6

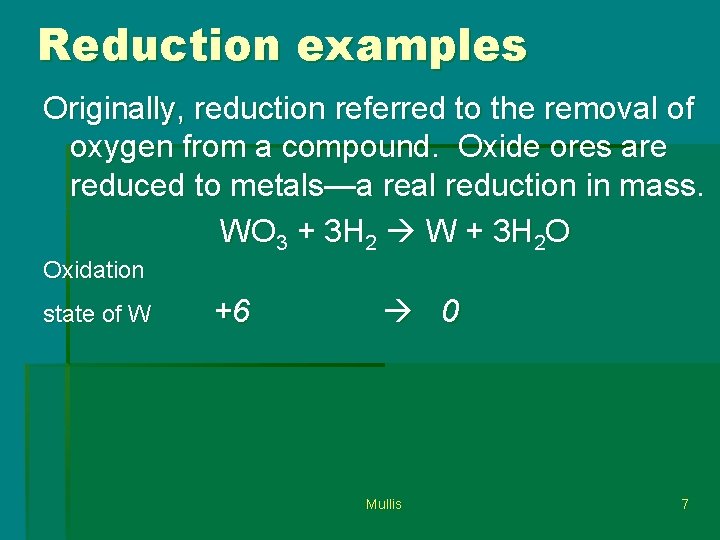
Reduction examples Originally, reduction referred to the removal of oxygen from a compound. Oxide ores are reduced to metals—a real reduction in mass. WO 3 + 3 H 2 W + 3 H 2 O Oxidation state of W +6 0 Mullis 7