Oxidation Reduction Red Ox Whats the point REDOX


















- Slides: 29

Oxidation - Reduction Red. Ox

What’s the point ? REDOX reactions are important in … C 3 H 8 O + Cr. O 3 + H 2 SO 4 Cr 2(SO 4)3 + C 3 H 6 O + H 2 O • Purifying metals (e. g. Al, Na, Li) • Producing gases (e. g. Cl 2, O 2, H 2) • Electroplating metals z Electrical production (batteries, fuel cells) • Protecting metals from corrosion • Balancing complex chemical equations • Sensors and machines (e. g. p. H meter)

Oxidation States z. . . of an element are determined from the number of electrons that are ______ other atoms y. Gained from y. Lost to AND y. Shared with

Rules of assigning oxidation states z. Atoms have a negative oxidation state if they have the higher e-neg in the bond y. NH 3 y. N=-3 y. H=+1 Oxidation states on all the atoms of a molecule & compound must add up to equal zero

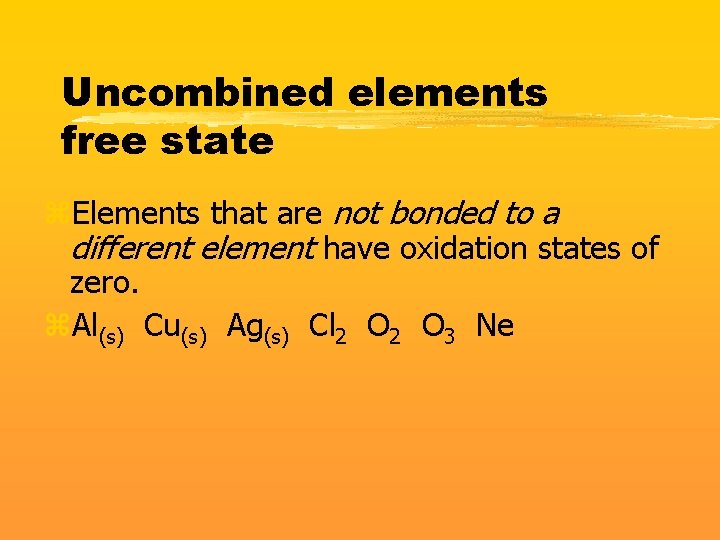
Uncombined elements free state z. Elements that are not bonded to a different element have oxidation states of zero. z. Al(s) Cu(s) Ag(s) Cl 2 O 3 Ne

NOW More complicated. . . z. H 2 S ysulfur has a -2 oxidation state z. H 2 SO 3 ysulfur has a +4 oxidation state z. H 2 SO 4 ysulfur has a +6 oxidation state

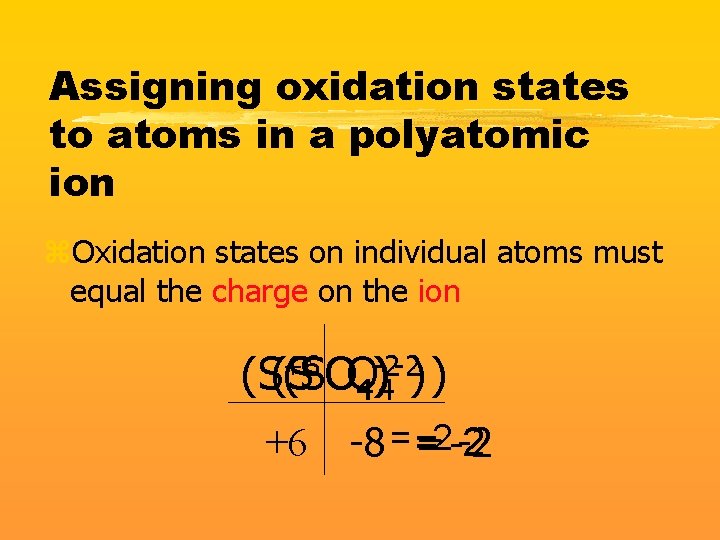
Assigning oxidation states to atoms in a polyatomic ion z. Oxidation states on individual atoms must equal the charge on the ion +6 -2 -2) ) (S(S (SO ) OO 44 +6 -2 -2 -8 = = = -2

Try these. . . zhydroxide zdichromate zammonium

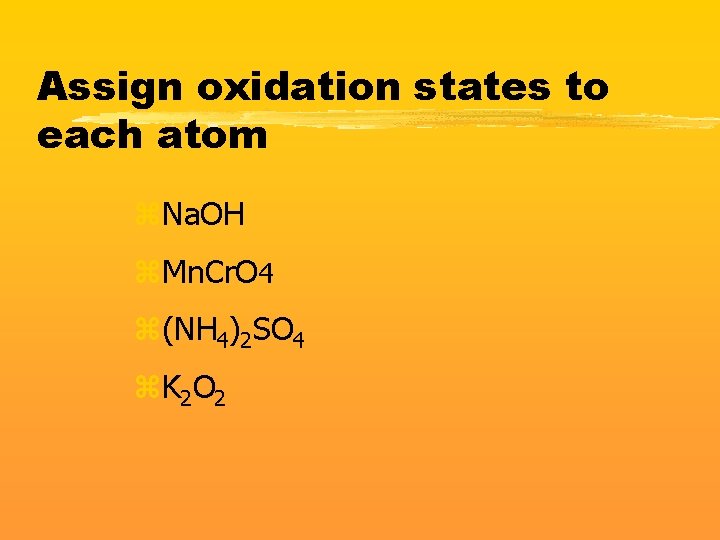
Assign oxidation states to each atom z. Na. OH z. Mn. Cr. O 4 z(NH 4)2 SO 4 z. K 2 O 2

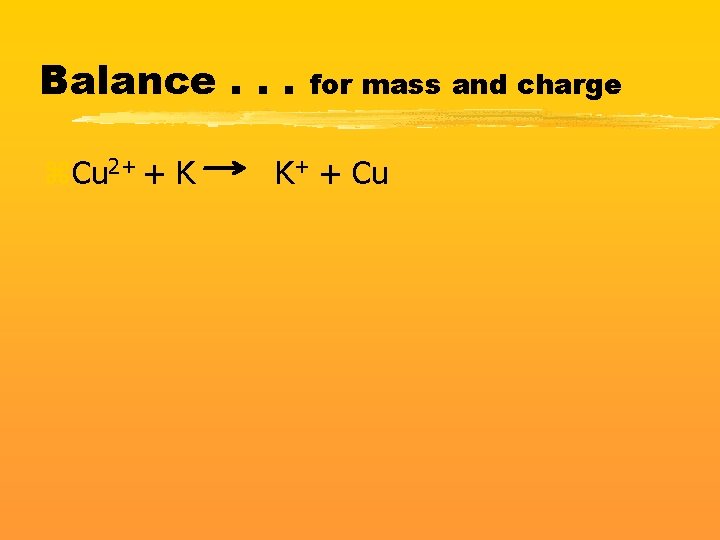
Balance. . . z. Cu 2+ + K for mass and charge K+ + Cu

Red. Ox reactions z. Chemical reactions involving an electron transfer between reactants

Recognizing Red. Ox zassign oxidation states to the individual elements in reactants and the products if the oxidation state changes for some of the particles it is considered a Red. Ox reaction Single Replacement Reactions areare ALWAYS Double Replacement Reactions NEVERRed. OX

In a Red. Ox reaction z. If one atom is being oxidized another must be reduced. z. In other words. . . yoxidation and reduction always occur together If electrons are lost by one species in a reaction they all MUST be gained by another!

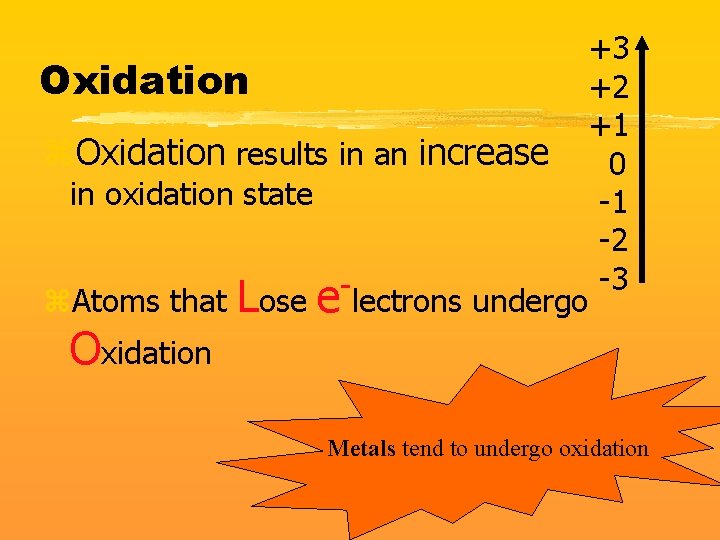
Oxidation z. Oxidation results in an increase in oxidation state z. Atoms that Lose Oxidation e-lectrons undergo +3 +2 +1 0 -1 -2 -3 Metals tend to undergo oxidation

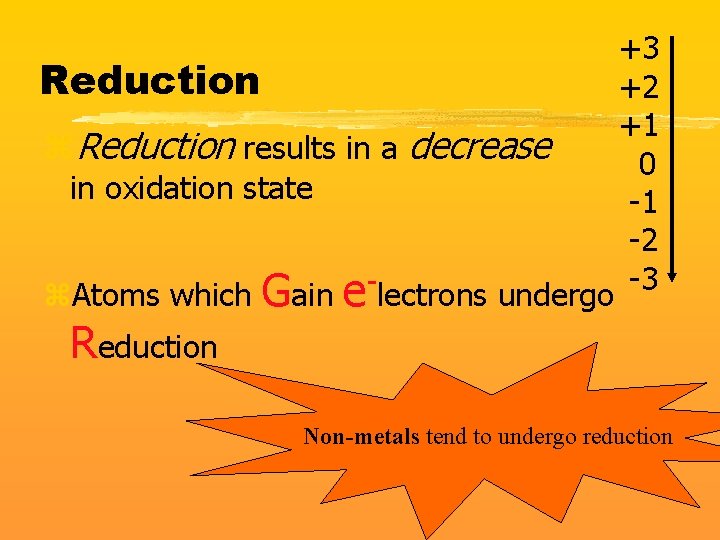
+3 Reduction +2 +1 z. Reduction results in a decrease 0 in oxidation state -1 -2 -3 z. Atoms which Gain e lectrons undergo Reduction Non-metals tend to undergo reduction

Ge. R

Red. Ox: Yes or NO? z. HCl + Na. OH HOH + Na. Cl z. Mg + 2 HCl Mg. Cl 2 + H 2 z. Mn. O 2 + 4 HBr Mn. Br 2 + 2 H 2 O

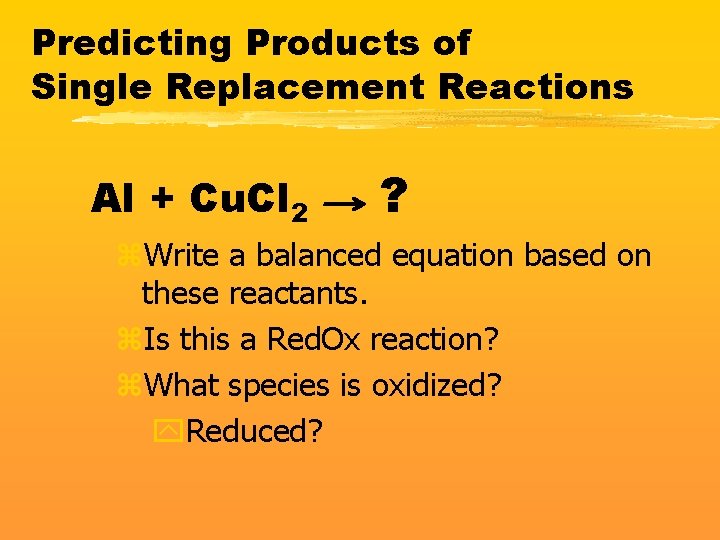
Predicting Products of Single Replacement Reactions Al + Cu. Cl 2 ? z. Write a balanced equation based on these reactants. z. Is this a Red. Ox reaction? z. What species is oxidized? y. Reduced?

Roaring Animals Often Attack z. Elements that undergo oxidation cause reduction y. Reducing agent z. Elements that undergo reduction cause oxidation y. Oxidizing agent LEO GER RA OA

2 Al + 3 Cu. Cl 2 2 Al. Cl 3 + 3 Cu z. What is the Oxidizing Agent? y. Cu+2 because it gets reduced z. What is the Reducing Agent? y. Al because it gets oxidized These answers always come from the reactant side

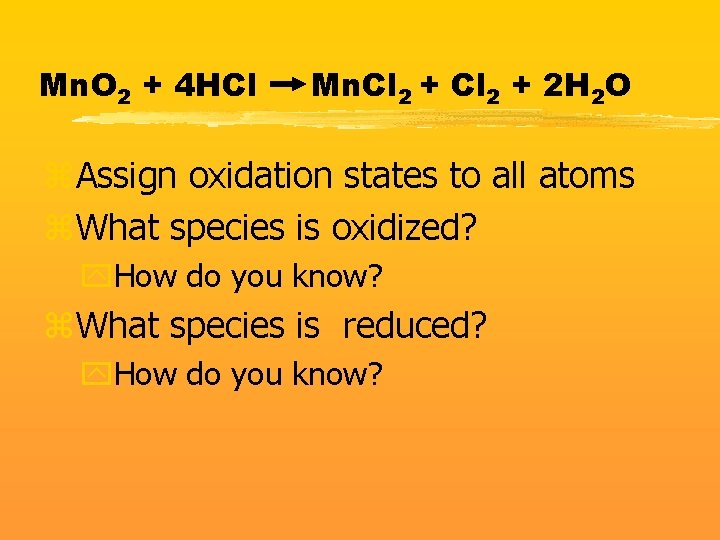
Mn. O 2 + 4 HCl Mn. Cl 2 + 2 H 2 O z. Assign oxidation states to all atoms z. What species is oxidized? y. How do you know? z. What species is reduced? y. How do you know?

z. What species is the oxidizing agent? y. Mn+4 because it gets reduced z. What species is the reducing agent? y. Cl- because it gets oxidized

- z. Remember. . . +4 +3 +2 +1 0 -1 -2 -3 -4 Gain e- Reduction Losing e oxidation Red. Ox Practice

Writing half-reactions z. A half reaction shows either oxidation or reduction of a Red. Ox reaction. z. The electrons being lost (oxidation) or gained (reduction) are also shown.

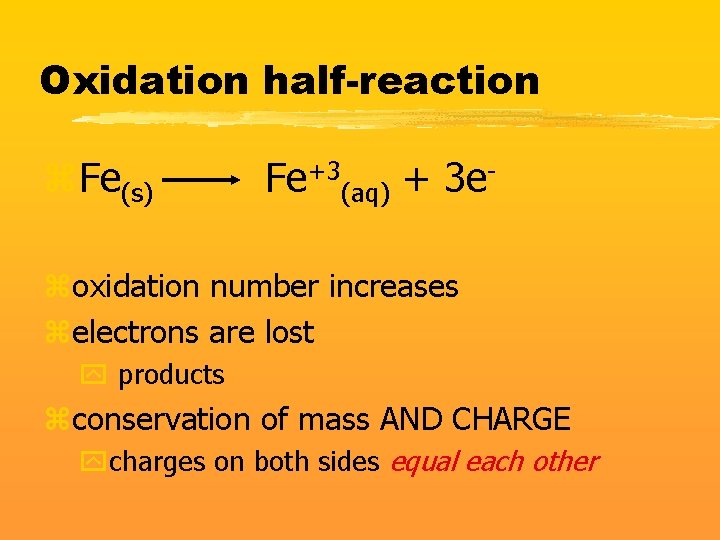
Oxidation half-reaction z. Fe(s) Fe+3(aq) + 3 e- zoxidation number increases zelectrons are lost y products zconservation of mass AND CHARGE ycharges on both sides equal each other

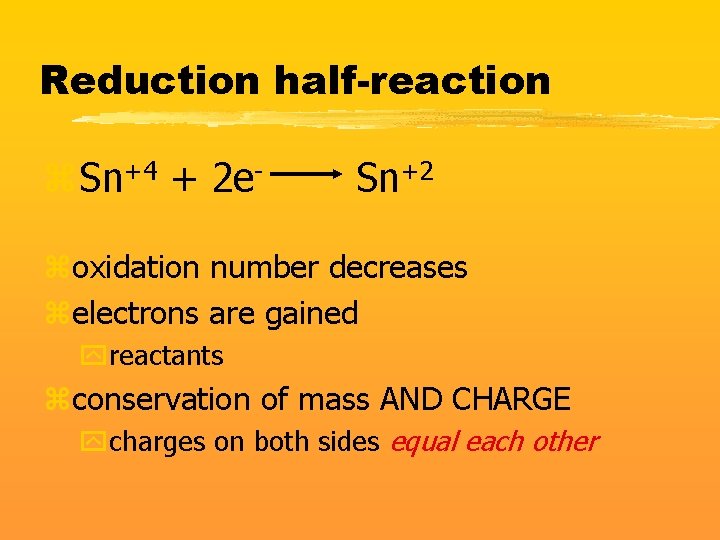
Reduction half-reaction z. Sn+4 + 2 e- Sn+2 zoxidation number decreases zelectrons are gained yreactants zconservation of mass AND CHARGE ycharges on both sides equal each other

Balancing Red. Ox using 1/2 reactions z 1. z 2. z 3. z 4. ASSIGN OXIDATION STATES. . . Write the oxidation 1/2 reaction Write the reduction 1/2 reaction Balance the two half reactions so that the number of electrons transferred is equal z 5. Use these coefficients to balance the Red. Ox atoms z 6. Balance leftover atoms by inventory

. . . use 1/2 reactions KMn. O 4 + HCl Mn. Cl 2 + KCl + Cl 2 + H 2 O Zn. S + O 2 SO 2 + Zn. O

The End I came. . . I saw. . . I Red. Oxed