Oxidation Reduction Ch 20 Intro to Redox Why









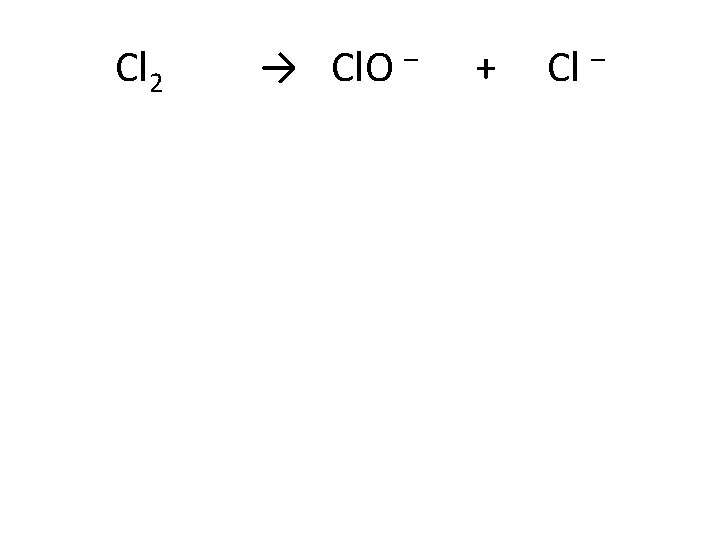
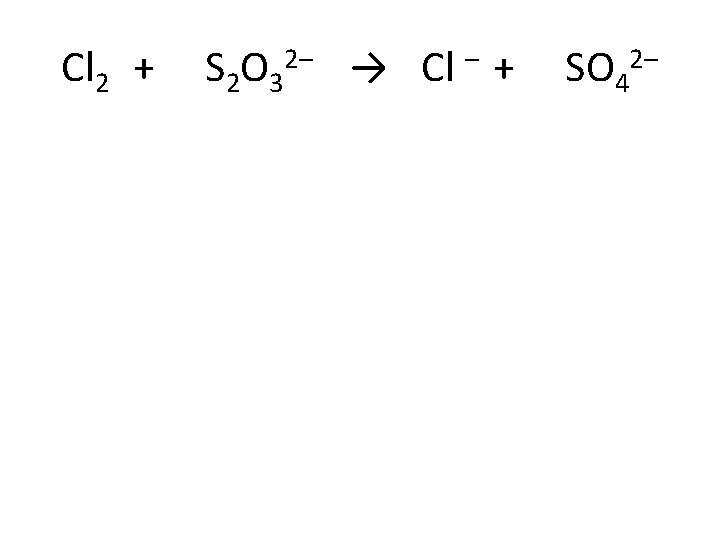
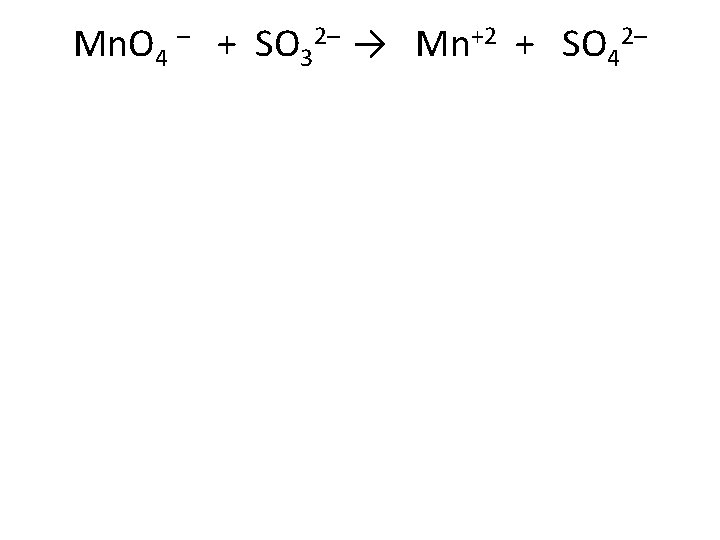
- Slides: 31

Oxidation & Reduction Ch 20

Intro to Redox Why do we call it “oxidation” ? ØIn the past scientists believed there was a gain or loss of oxygen. This is FALSE!

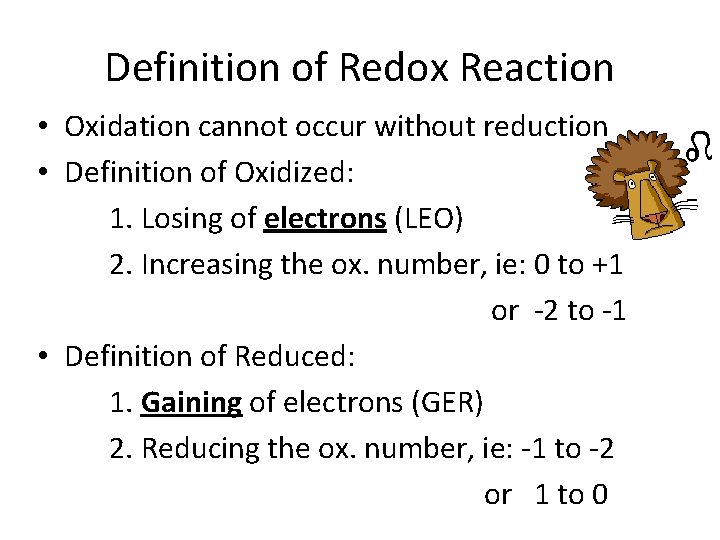
Definition of Redox Reaction ØOxidation Reduction Reaction (Redox): A reaction in which electrons are transferred from one substance to another.

Definition of Redox Reaction • Oxidation cannot occur without reduction • Definition of Oxidized: 1. Losing of electrons (LEO) 2. Increasing the ox. number, ie: 0 to +1 or -2 to -1 • Definition of Reduced: 1. Gaining of electrons (GER) 2. Reducing the ox. number, ie: -1 to -2 or 1 to 0

Redox Rules to memorize! The Statue of Liberty’s outer layer of copper has undergone redox

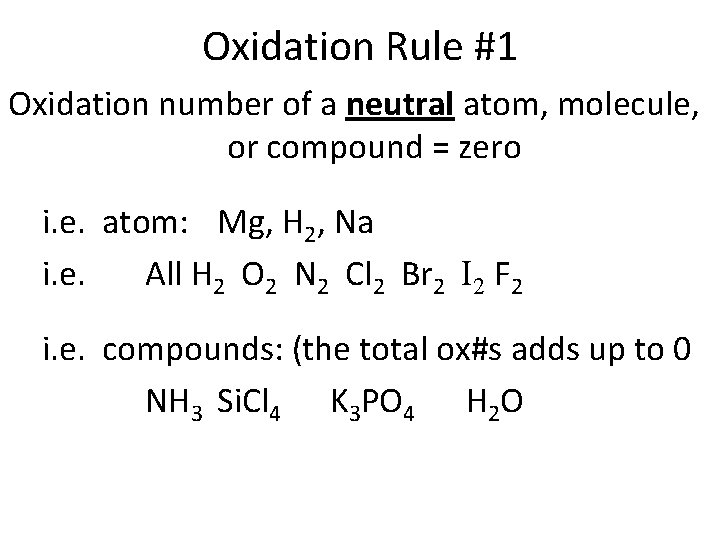
Oxidation Rule #1 Oxidation number of a neutral atom, molecule, or compound = zero i. e. atom: Mg, H 2, Na i. e. All H 2 O 2 N 2 Cl 2 Br 2 I 2 F 2 i. e. compounds: (the total ox#s adds up to 0 NH 3 Si. Cl 4 K 3 PO 4 H 2 O

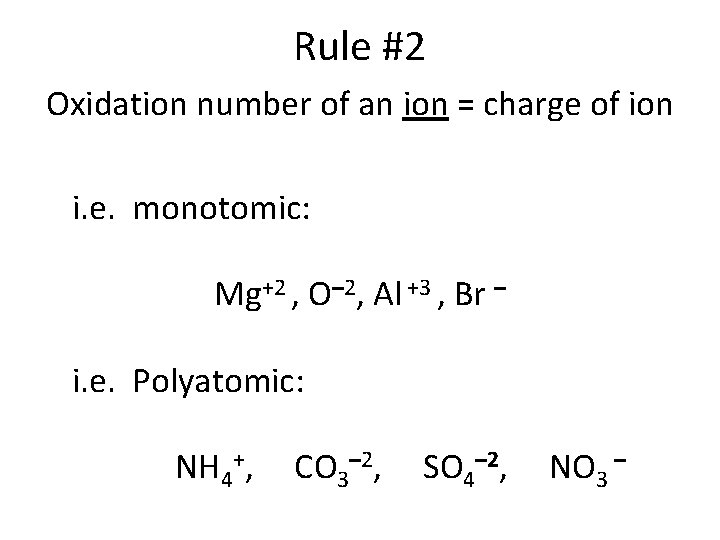
Rule #2 Oxidation number of an ion = charge of ion i. e. monotomic: Mg+2 , O– 2, Al +3 , Br – i. e. Polyatomic: NH 4+, CO 3– 2, SO 4– 2, NO 3 –

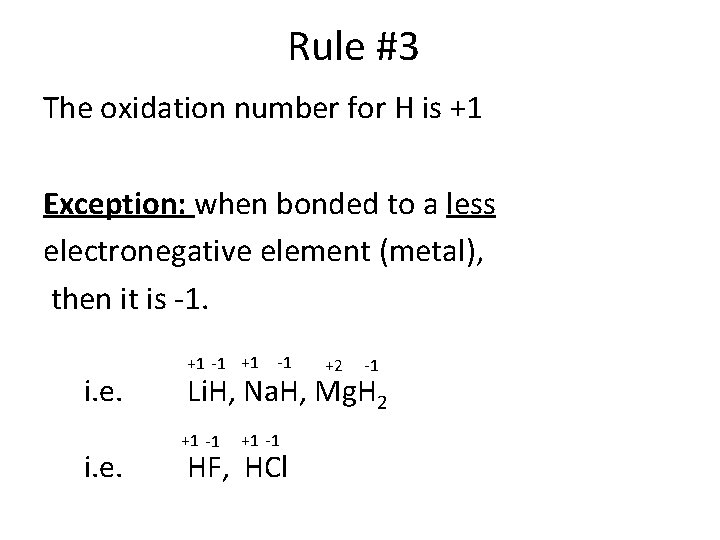
Rule #3 The oxidation number for H is +1 Exception: when bonded to a less electronegative element (metal), then it is -1. i. e. +1 -1 +2 -1 Li. H, Na. H, Mg. H 2 +1 -1 HF, HCl

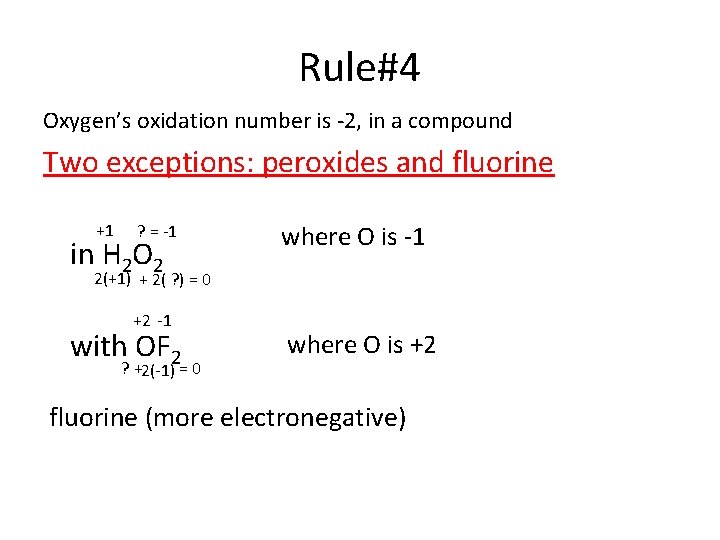
Rule#4 Oxygen’s oxidation number is -2, in a compound Two exceptions: peroxides and fluorine +1 ? = -1 in H 2 O 2 where O is -1 2(+1) + 2( ? ) = 0 +2 -1 with OF 2 ? +2(-1) = 0 where O is +2 fluorine (more electronegative)

Rule #5 • The most electronegative element fluorine ALWAYS has an oxidation number of -1 when it is bonded to another element. • i. e. HF

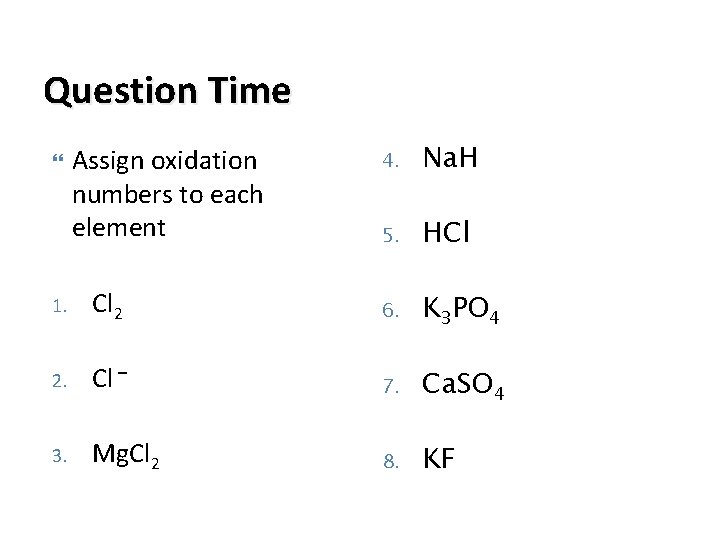
Question Time Assign oxidation numbers to each element 4. Na. H 5. HCl 1. Cl 2 6. K 3 PO 4 2. Cl – 7. Ca. SO 4 3. Mg. Cl 2 8. KF

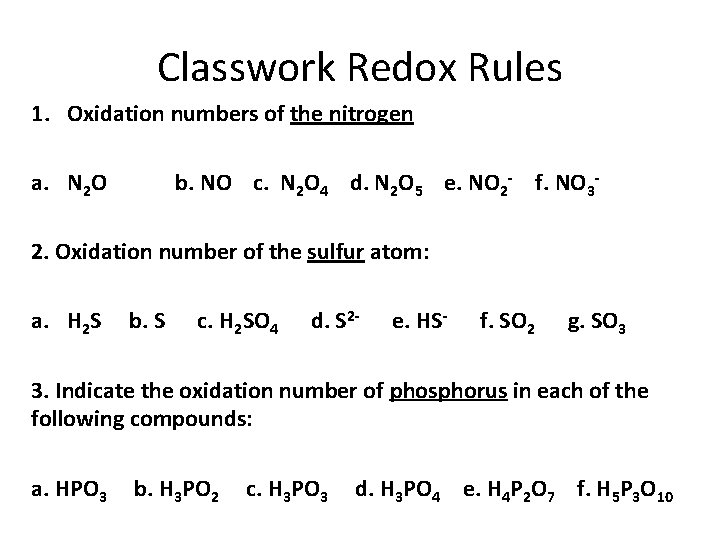
Classwork Redox Rules 1. Oxidation numbers of the nitrogen a. N 2 O b. NO c. N 2 O 4 d. N 2 O 5 e. NO 2 - f. NO 3 - 2. Oxidation number of the sulfur atom: a. H 2 S b. S c. H 2 SO 4 d. S 2 - e. HS- f. SO 2 g. SO 3 3. Indicate the oxidation number of phosphorus in each of the following compounds: a. HPO 3 b. H 3 PO 2 c. H 3 PO 3 d. H 3 PO 4 e. H 4 P 2 O 7 f. H 5 P 3 O 10

Half Reactions

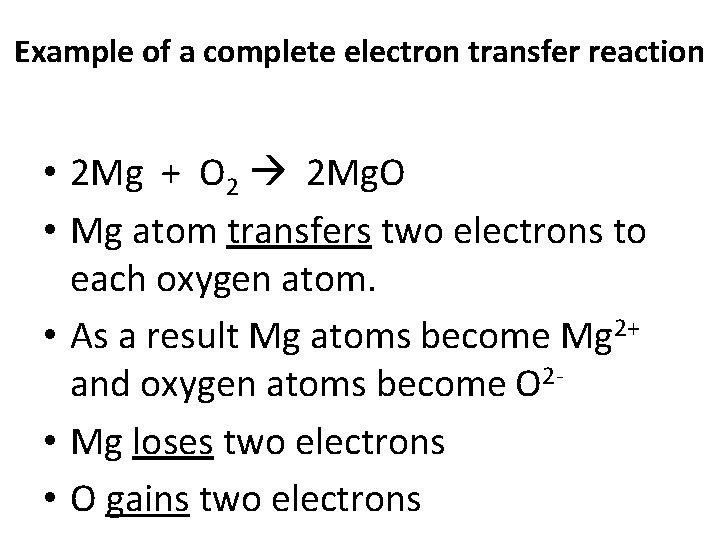
Example of a complete electron transfer reaction • 2 Mg + O 2 2 Mg. O • Mg atom transfers two electrons to each oxygen atom. • As a result Mg atoms become Mg 2+ and oxygen atoms become O 2 • Mg loses two electrons • O gains two electrons

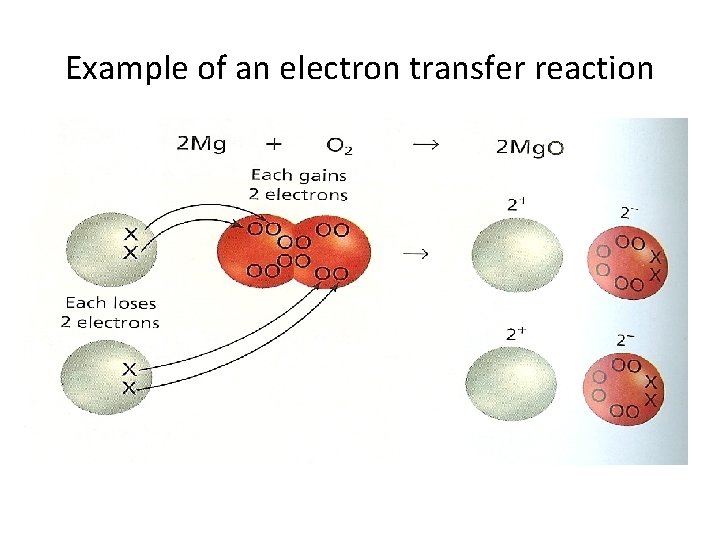
Example of an electron transfer reaction • 2 Mg + O 2 2 Mg. O • Mg atom transfers two electrons to each oxygen atom. • As a result 2 Mg atoms become Mg 2+ and two oxygen atoms become O 2 • Mg loses two electrons • O gains two electrons

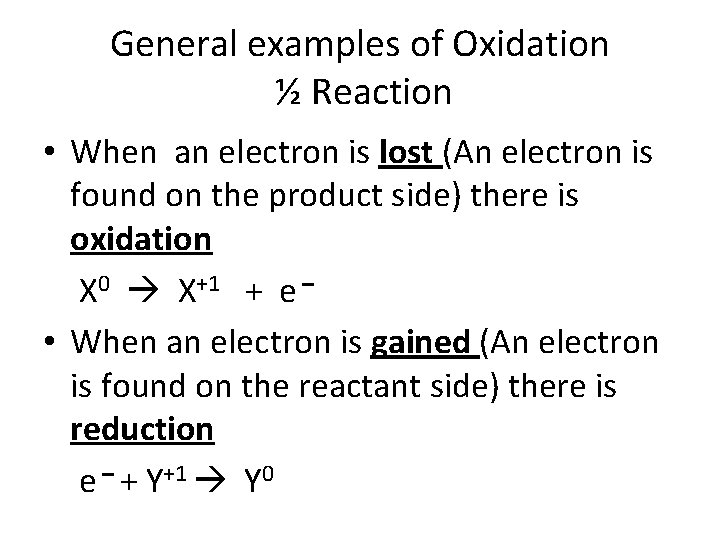
General examples of Oxidation ½ Reaction • When an electron is lost (An electron is found on the product side) there is oxidation X 0 X+1 + e – • When an electron is gained (An electron is found on the reactant side) there is reduction e – + Y+1 Y 0

Using the Rules Oxidation Number • If oxidation number goes up it is oxidized. • If oxidation number goes down it is reduced.

Two ways to remember oxidation/reduction (redox) L E O goes G E R O S E L E C T R O N S X I D A T I O N A I N L E C T R O N S E D U C T I O N O I L R I G X I D A T I O N S O S I N G E D U C T I O N S A I N G

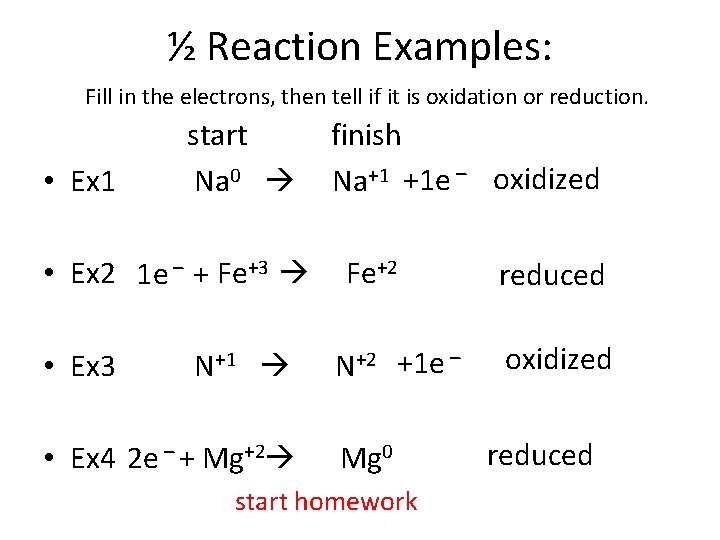
½ Reaction Examples: Fill in the electrons, then tell if it is oxidation or reduction. • Ex 1 start Na 0 • Ex 2 1 e – + Fe+3 • Ex 3 N+1 • Ex 4 2 e – + Mg+2 finish Na+1 +1 e – oxidized Fe+2 N+2 +1 e – Mg 0 start homework reduced oxidized reduced

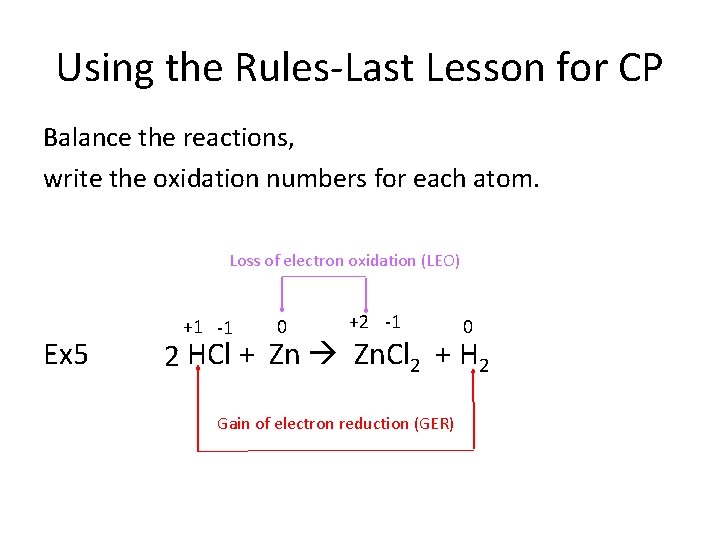
Using the Rules-Last Lesson for CP Balance the reactions, write the oxidation numbers for each atom. Loss of electron oxidation (LEO) Ex 5 +1 -1 0 +2 -1 0 2 HCl + Zn Zn. Cl 2 + H 2 Gain of electron reduction (GER)

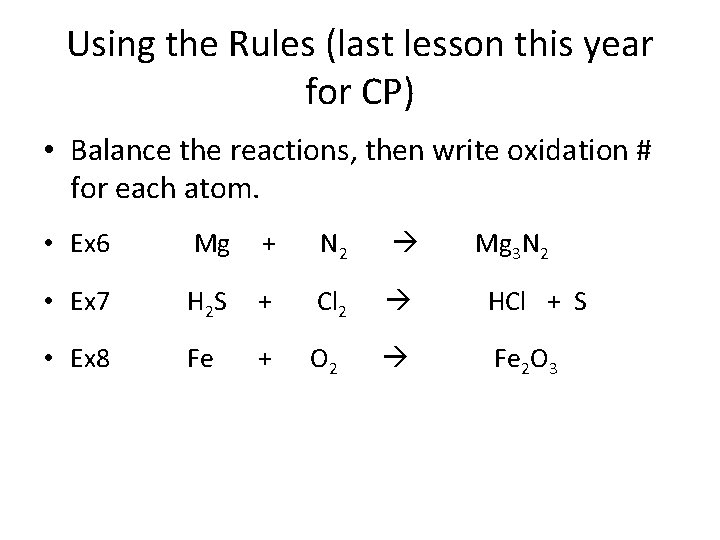
Using the Rules (last lesson this year for CP) • Balance the reactions, then write oxidation # for each atom. • Ex 6 Mg + N 2 Mg 3 N 2 • Ex 7 H 2 S + Cl 2 HCl + S • Ex 8 Fe + O 2 Fe 2 O 3

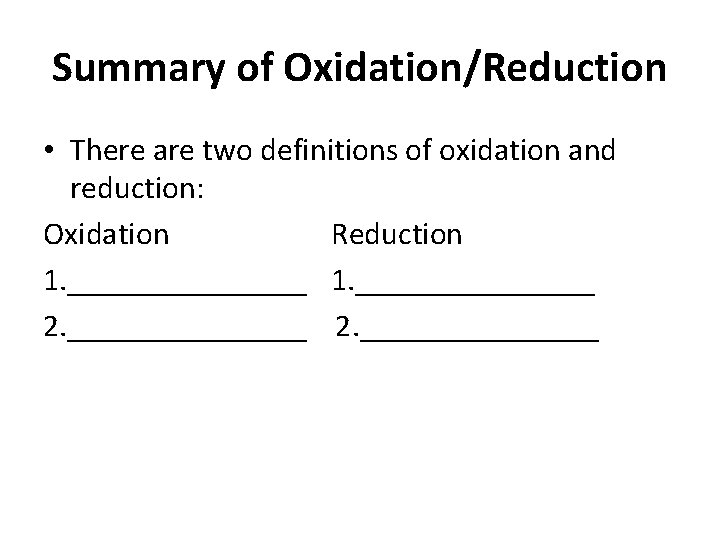
Summary of Oxidation/Reduction • There are two definitions of oxidation and reduction: Oxidation Reduction 1. _______________ 2. _______________

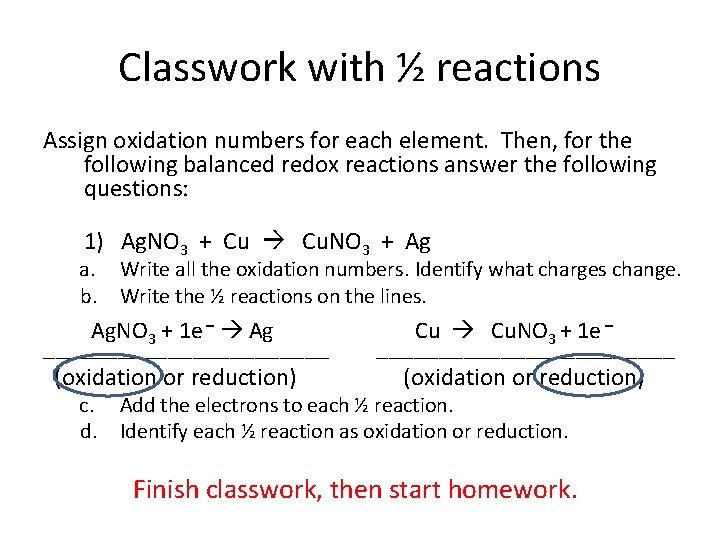
Classwork with ½ reactions Assign oxidation numbers for each element. Then, for the following balanced redox reactions answer the following questions: 1) Ag. NO 3 + Cu Cu. NO 3 + Ag a. Write all the oxidation numbers. Identify what charges change. b. Write the ½ reactions on the lines. Ag. NO 3 + 1 e – Ag ____________ (oxidation or reduction) Cu Cu. NO 3 + 1 e – ____________ (oxidation or reduction) c. Add the electrons to each ½ reaction. d. Identify each ½ reaction as oxidation or reduction. Finish classwork, then start homework.

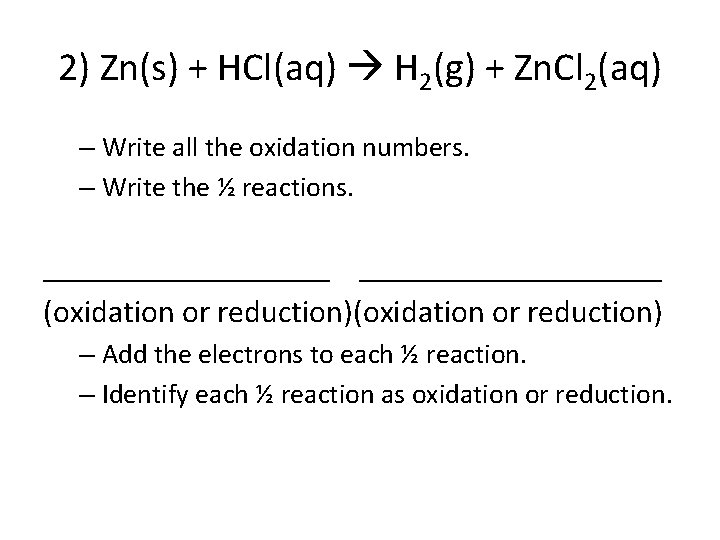
2) Zn(s) + HCl(aq) H 2(g) + Zn. Cl 2(aq) – Write all the oxidation numbers. – Write the ½ reactions. ___________________ (oxidation or reduction) – Add the electrons to each ½ reaction. – Identify each ½ reaction as oxidation or reduction.

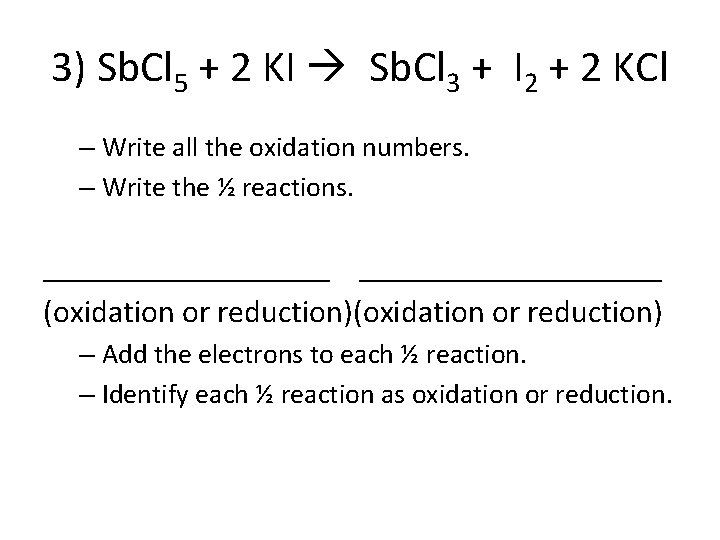
3) Sb. Cl 5 + 2 KI Sb. Cl 3 + I 2 + 2 KCl – Write all the oxidation numbers. – Write the ½ reactions. ___________________ (oxidation or reduction) – Add the electrons to each ½ reaction. – Identify each ½ reaction as oxidation or reduction.

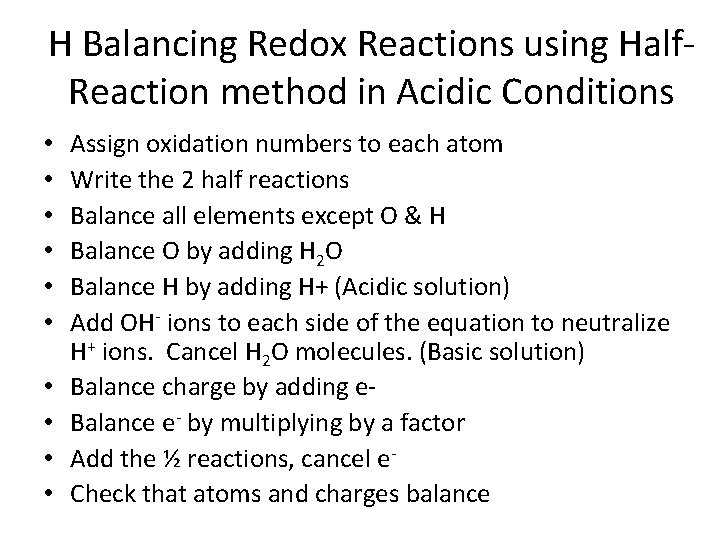
H Balancing Redox Reactions using Half. Reaction method in Acidic Conditions • • • Assign oxidation numbers to each atom Write the 2 half reactions Balance all elements except O & H Balance O by adding H 2 O Balance H by adding H+ (Acidic solution) Add OH- ions to each side of the equation to neutralize H+ ions. Cancel H 2 O molecules. (Basic solution) Balance charge by adding e. Balance e- by multiplying by a factor Add the ½ reactions, cancel e. Check that atoms and charges balance

Ex 1: Mn. O 4 – + Fe+2 → Fe+3 + Mn+2
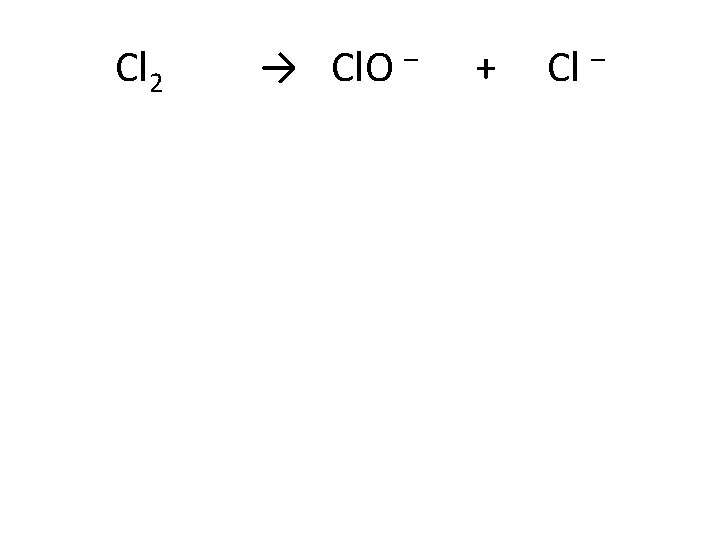
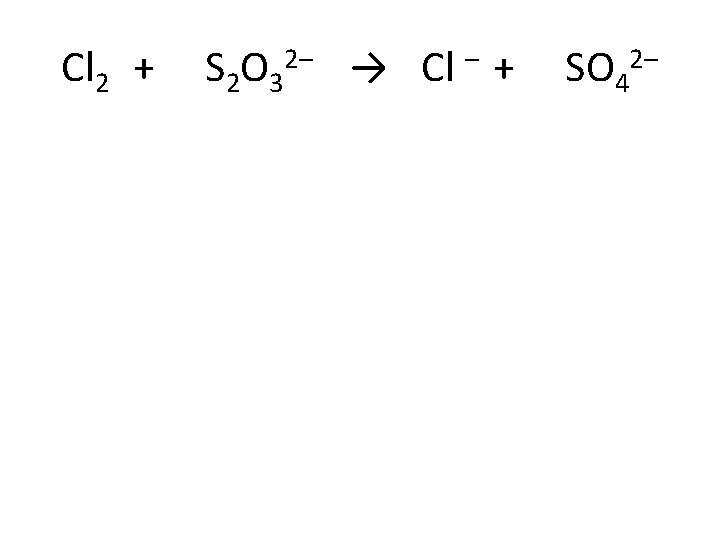
Cl 2 + S 2 O 32– → Cl – + SO 42–
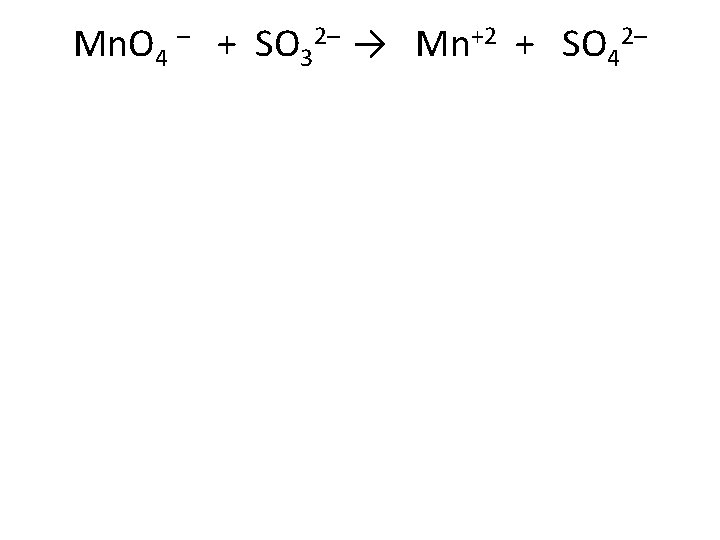
Mn. O 4 – + SO 32– → Mn+2 + SO 42–