Oxidation Reduction and Electrochemistry TOPIC 1 Half Reactions









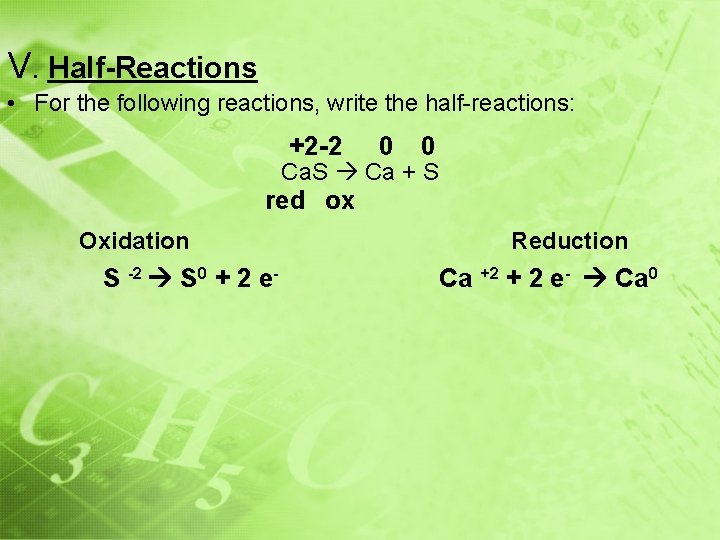



- Slides: 38

Oxidation, Reduction, and Electrochemistry

TOPIC 1: Half Reactions Objective: Determine oxidation numbers for elements in a compound, identify the oxidized and reduced species, write half reaction, and balance ionic reactions

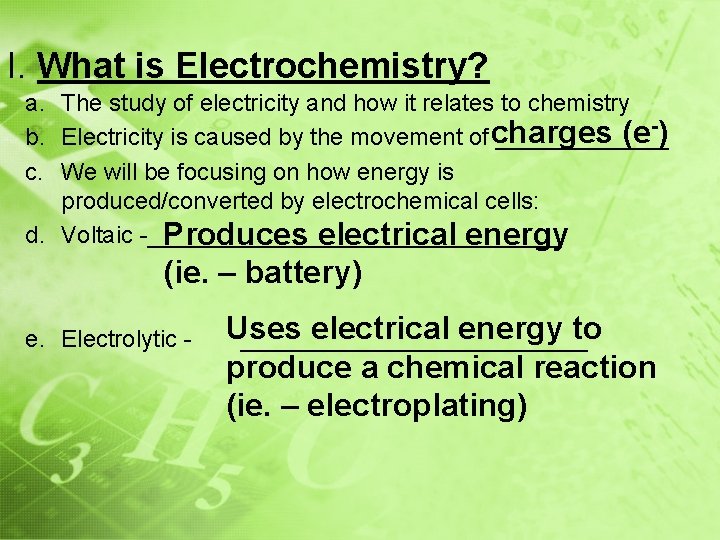
I. What is Electrochemistry? a. The study of electricity and how it relates to chemistry (e-) b. Electricity is caused by the movement of charges _______ c. We will be focusing on how energy is produced/converted by electrochemical cells: d. Voltaic -________________ Produces electrical energy (ie. – battery) e. Electrolytic - Uses electrical energy to _____________ produce a chemical reaction (ie. – electroplating)

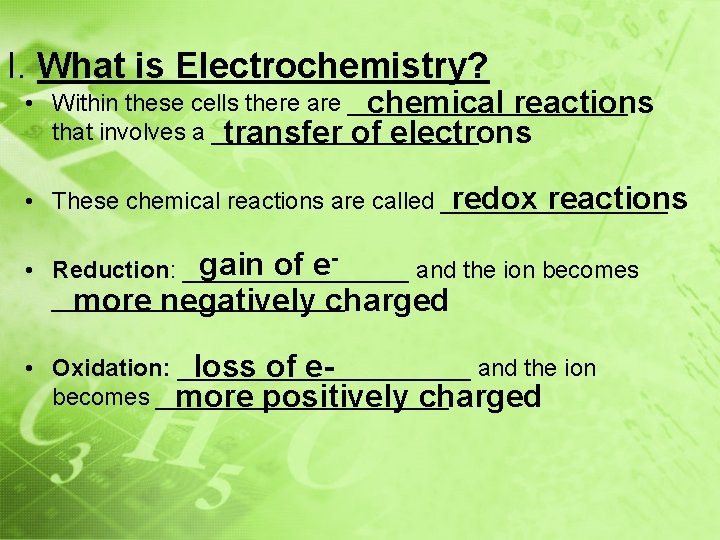
I. What is Electrochemistry? • Within these cells there are ___________ chemical reactions that involves a __________ transfer of electrons redox reactions • These chemical reactions are called _________ gain of e • Reduction: _________ and the ion becomes ___________ more negatively charged - • Oxidation: ___________ and the ion loss of ebecomes ___________ more positively charged

I. What is Electrochemistry? • Way to remember it: “LEO says GER” LOSS of Electrons is OXIDATION GAIN of Electrons is REDUCTION

II. Assigning Oxidation Numbers • The first step in determining which species was oxidized and which was reduced is knowing what the charge of each species starts at and ends at, so you can determine whether electrons were lost or gained. Why is that important? • Ever put a battery in backwards? It doesn’t work! Circuits are designed to only let electrons through ONE way. It is possible to destroy an electronic device by putting the battery in backwards. We need to know which end is – (the end that loses electrons, oxidation) and which end is + (the end that gains electrons, reduction), so we need to know how to find the charges (oxidation numbers) of the species involved!

II. Assigning Oxidation Numbers • Rules for assigning oxidation number (REVIEW): 1. Each uncombined element has an oxidation number zero of _____ • Example: 2 Na + Cl 2 2 Na. Cl _________ has an oxidation number (charge) of Na and Cl 2 _______ zero 2. If an element has only ______ one oxidation number (charge) listed on the __________, periodic table then that is its oxidation number +1 • Examples: Na has a charge of _____ Ca has a charge of _____ +2 Al has a charge of _____ +3 -2 O has a charge of _____

II. Assigning Oxidation Numbers • Rules for assigning oxidation number (REVIEW): ZERO 3. The sum of all charges in an compound equals _____ Examples: Show that the sum of the charges equals zero for the following compounds: Na. Cl 1(+1) + 1(-1) = 0 Li 3 N 3(+1) + 1(-3) = 0 Mg. Cl 2 1(+2) + 2(-1) = 0

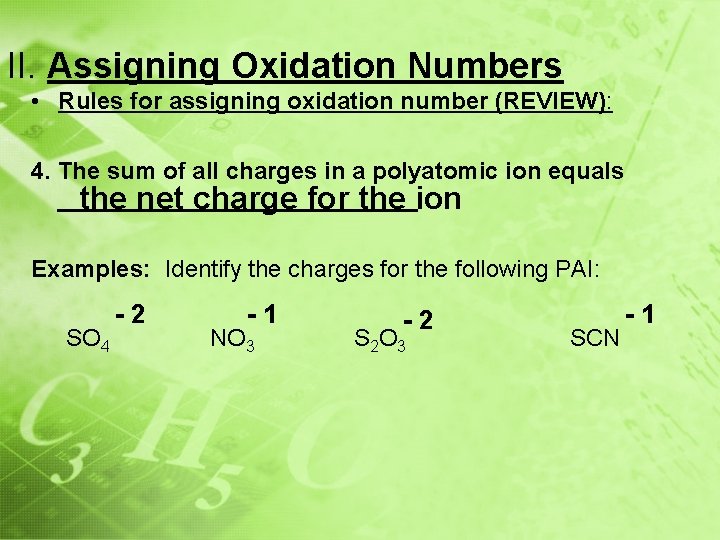
II. Assigning Oxidation Numbers • Rules for assigning oxidation number (REVIEW): 4. The sum of all charges in a polyatomic ion equals ______________ the net charge for the ion Examples: Identify the charges for the following PAI: SO 4 -2 -1 NO 3 -2 S 2 O 3 SCN -1

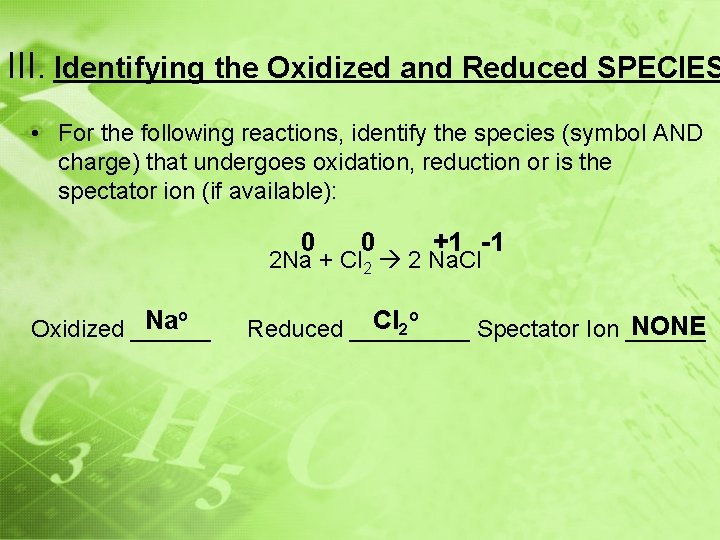
III. Identifying the Oxidized and Reduced SPECIES • If the charge becomes more positive going from left to OXIDIZED and ________ right, the species is _____ LOSES ELECTRONS (OX # ↑) • If the charge becomes more negative going from left to right, the species is REDUCED _____ and _________ GAINS ELECTRONS (OX # ↓) ION THAT DOES NOT • Spectator Ions _________________ CHANGE CHARGE

III. Identifying the Oxidized and Reduced SPECIES • For the following reactions, identify the species (symbol AND charge) that undergoes oxidation, reduction or is the spectator ion (if available): 0 0 +1 -1 2 Na + Cl 2 2 Na. Cl o Na Oxidized ______ o Cl NONE 2 Reduced _____ Spectator Ion ______

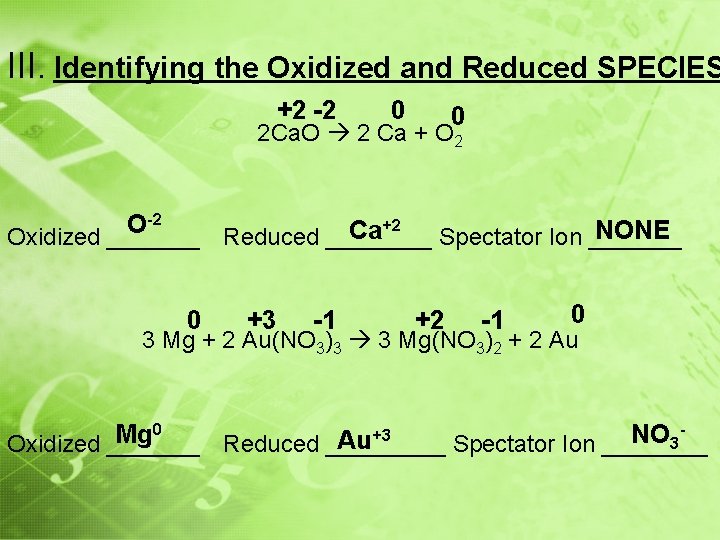
III. Identifying the Oxidized and Reduced SPECIES +2 -2 0 0 2 Ca. O 2 Ca + O 2 -2 +2 O Ca NONE Oxidized _______ Reduced ____ Spectator Ion _______ 0 +3 -1 +2 -1 0 3 Mg + 2 Au(NO 3)3 3 Mg(NO 3)2 + 2 Au 0 +3 Mg NO Au 3 Oxidized _______ Reduced _____ Spectator Ion ____

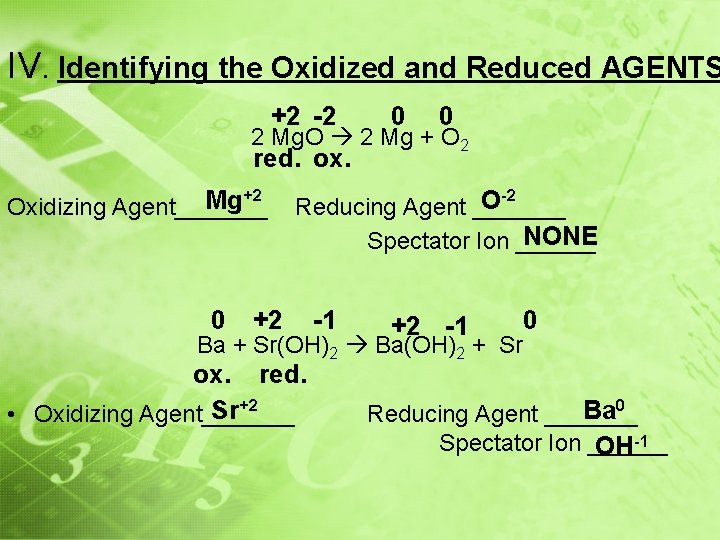
IV. Identifying the Oxidized and Reduced AGENTS reduction (or is _______) • Reducing Agent: causes ______ oxidized oxidation (or is _______) reduced • Oxidizing Agent: causes ______ **AGENTS ALWAYS COME FROM THE REACTANT SIDE** • For the following reactions, identify the oxidizing and reducing agent and spectator ion (if possible) 0 0 +1 -1 2 Li + F 2 2 Li. F ox. red. F 20 Reducing Agent _____ Li 0 • Oxidizing Agent _____ NONE Spectator Ion ______

IV. Identifying the Oxidized and Reduced AGENTS +2 -2 0 0 2 Mg. O 2 Mg + O 2 red. ox. Mg Oxidizing Agent_______ +2 0 O Reducing Agent _______ NONE Spectator Ion ______ +2 -2 -1 +2 -1 Ba + Sr(OH)2 Ba(OH)2 + Sr ox. red. Sr+2 • Oxidizing Agent_______ 0 Ba 0 Reducing Agent _______ Spectator Ion ______ OH-1

Identifying Redox Reactions • (Most) Redox reactions have a free element on one side of a reaction and the same element bonded to something else on the other side • Examples: • 2 H 2 + O 2 --> 2 H 2 O • 2 KCl. O 3 --> 2 KCl +3 O 2 Free element ---> Bonded element Bonded oxygen ----> Free oxygen

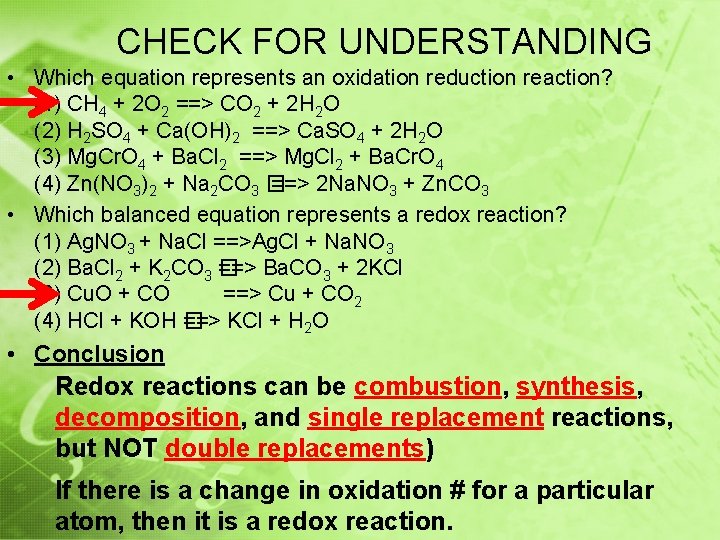
CHECK FOR UNDERSTANDING • Which equation represents an oxidation reduction reaction? (1) CH 4 + 2 O 2 ==> CO 2 + 2 H 2 O (2) H 2 SO 4 + Ca(OH)2 ==> Ca. SO 4 + 2 H 2 O (3) Mg. Cr. O 4 + Ba. Cl 2 ==> Mg. Cl 2 + Ba. Cr. O 4 (4) Zn(NO 3)2 + Na 2 CO 3 � ==> 2 Na. NO 3 + Zn. CO 3 • Which balanced equation represents a redox reaction? (1) Ag. NO 3 + Na. Cl ==>Ag. Cl + Na. NO 3 (2) Ba. Cl 2 + K 2 CO 3 � ==> Ba. CO 3 + 2 KCl (3) Cu. O + CO ==> Cu + CO 2 (4) HCl + KOH � ==> KCl + H 2 O • Conclusion Redox reactions can be combustion, synthesis, decomposition, and single replacement reactions, but NOT double replacements) If there is a change in oxidation # for a particular atom, then it is a redox reaction.

V. Half-Reactions • A redox reaction may be split into 2 half-reactions, one for oxidation reduction ________ and the other for __________ • Oxidation: X 0 X+ + e. X- X 0 + e • Reduction: X+ + e- X 0 + e - X (e- on the _______ side product lost shows that it has been _______) reactant side (e- on the ______ gained shows that it has been_______) IN GENERAL: e- go on the more positive side
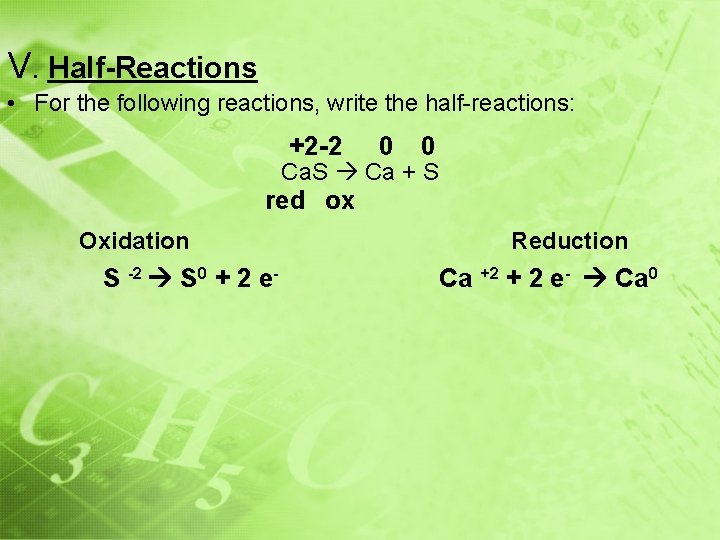
V. Half-Reactions • For the following reactions, write the half-reactions: +2 -2 0 0 Ca. S Ca + S red ox Oxidation S -2 S 0 + 2 e- Reduction Ca +2 + 2 e- Ca 0

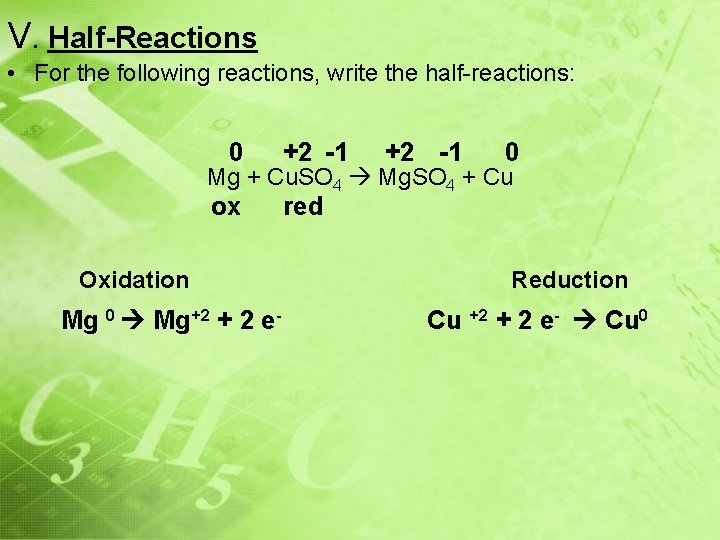
V. Half-Reactions • For the following reactions, write the half-reactions: 0 +2 -1 0 Mg + Cu. SO 4 Mg. SO 4 + Cu ox Oxidation Mg 0 Mg+2 + 2 e- red Reduction Cu +2 + 2 e- Cu 0

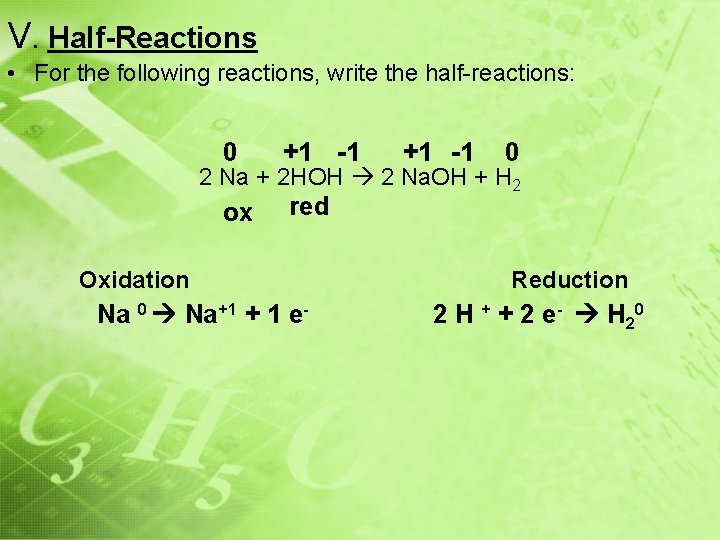
V. Half-Reactions • For the following reactions, write the half-reactions: 0 +1 -1 ox red +1 -1 0 2 Na + 2 HOH 2 Na. OH + H 2 Oxidation Na 0 Na+1 + 1 e- Reduction 2 H + + 2 e - H 20

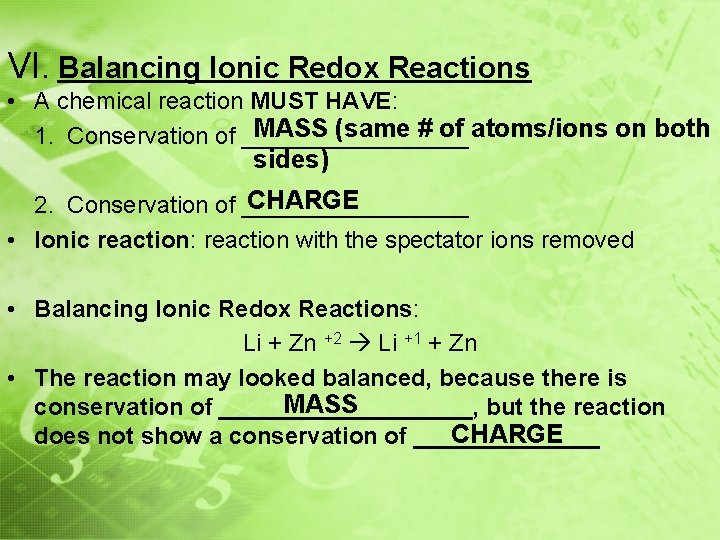
VI. Balancing Ionic Redox Reactions • A chemical reaction MUST HAVE: MASS (same # of atoms/ions on both 1. Conservation of _________ sides) CHARGE 2. Conservation of _________ • Ionic reaction: reaction with the spectator ions removed • Balancing Ionic Redox Reactions: Li + Zn +2 Li +1 + Zn • The reaction may looked balanced, because there is MASS conservation of __________, but the reaction CHARGE does not show a conservation of _______

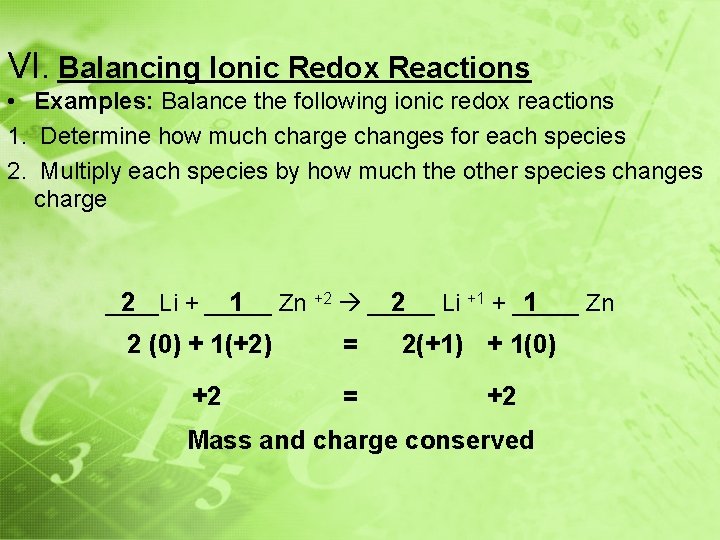
VI. Balancing Ionic Redox Reactions • Examples: Balance the following ionic redox reactions 1. Determine how much charge changes for each species 2. Multiply each species by how much the other species changes charge 2 1 Zn +2 _____ 2 Li +1 + _____ 1 ____Li + _____ Zn 2 (0) + 1(+2) = +2 = 2(+1) + 1(0) +2 Mass and charge conserved

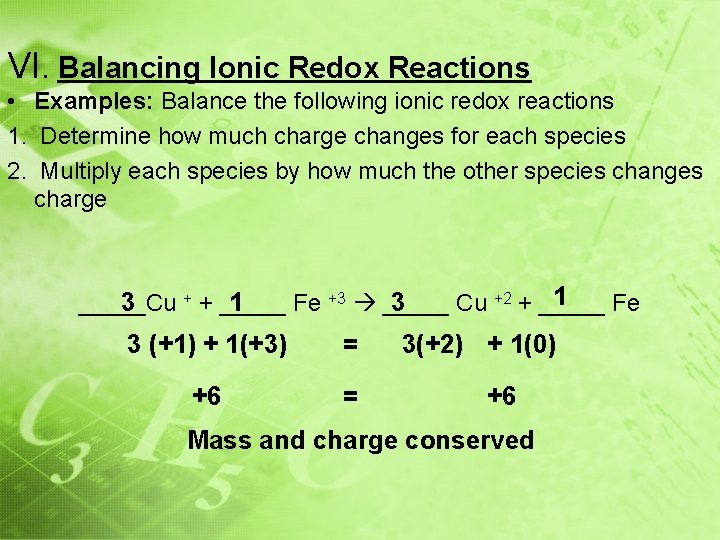
VI. Balancing Ionic Redox Reactions • Examples: Balance the following ionic redox reactions 1. Determine how much charge changes for each species 2. Multiply each species by how much the other species changes charge + + _____ 1 3 _____Cu Fe +3 _____ Cu +2 + _____ Fe 3 (+1) + 1(+3) = +6 = 3(+2) + 1(0) +6 Mass and charge conserved

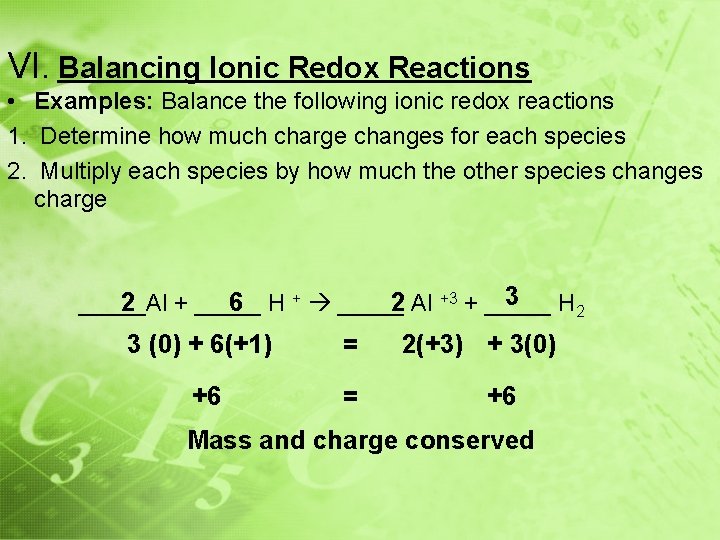
VI. Balancing Ionic Redox Reactions • Examples: Balance the following ionic redox reactions 1. Determine how much charge changes for each species 2. Multiply each species by how much the other species changes charge 3 H 2 2 + _____ 6 H + _____ 2 Al +3 + _____Al 3 (0) + 6(+1) = +6 = 2(+3) + 3(0) +6 Mass and charge conserved

TOPIC 2: Electrochemistry Objective: Predict which redox reactions will be spontaneous based on their position on the activity series table, identify the parts of a voltaic and electrolytic cell and describe their purpose, explain why the changes in concentration in each half cell are occurring as the reaction proceeds, and design a voltaic and electrolytic cell of your own.

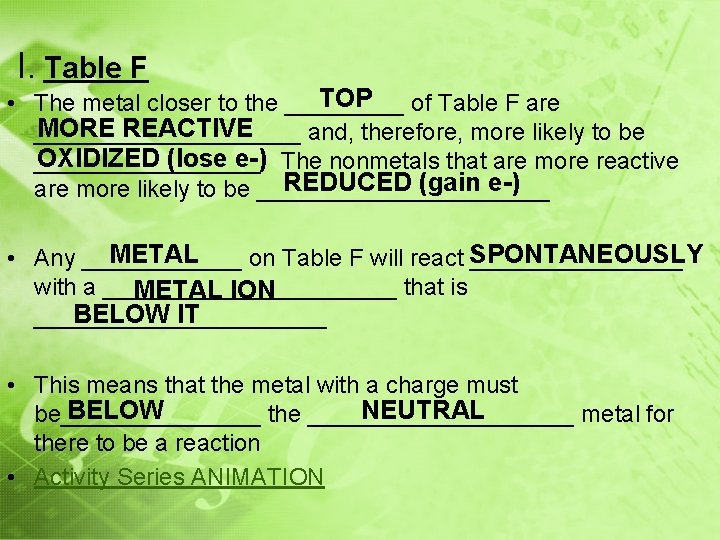
I. Table F TOP of Table F are • The metal closer to the _____ MORE REACTIVE __________ and, therefore, more likely to be OXIDIZED (lose e-) The nonmetals that are more reactive _________. REDUCED (gain e-) are more likely to be ___________ METAL • Any ______ on Table F will react SPONTANEOUSLY ________ with a ___________ that is METAL ION BELOW IT ___________ • This means that the metal with a charge must BELOW NEUTRAL be________ the __________ metal for there to be a reaction • Activity Series ANIMATION

I. Table F • Example: According to Table J, which of the following will react spontaneously? X + H + H 2 + X + A) Pb +2 B) Sn+2 C) Fe D) H 2 • Example: Which of the following will replace Ni+2 in the compound Ni(NO 3)2? A) Sn +2 B) Pb+2 C) Sn D) Cr • Example: Which atom/ion pair will Co oxidize spontaneously under standard conditions? A) Co + Fe+2 B) Co + Pb+2 C) Co + Cu D) Co + Cr+3

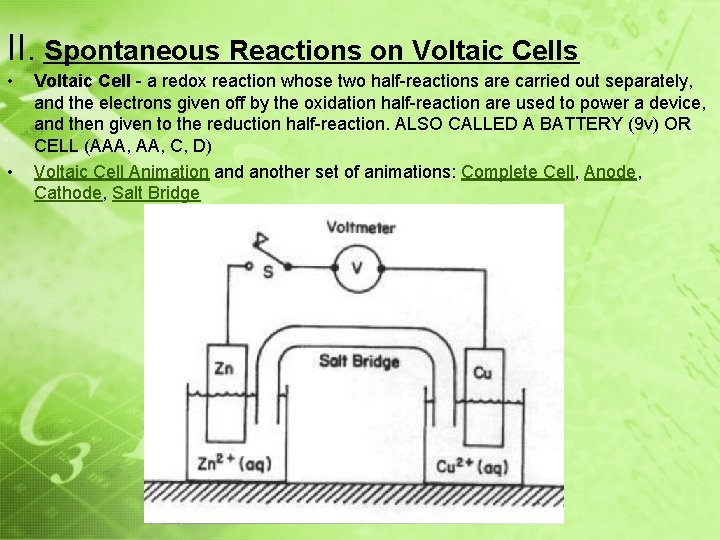
II. Spontaneous Reactions on Voltaic Cells • • Voltaic Cell - a redox reaction whose two half-reactions are carried out separately, and the electrons given off by the oxidation half-reaction are used to power a device, and then given to the reduction half-reaction. ALSO CALLED A BATTERY (9 v) OR CELL (AAA, C, D) Voltaic Cell Animation and another set of animations: Complete Cell, Anode, Cathode, Salt Bridge • How a Voltaic Wet Cell Works: CHEMICAL energy to _______ ELECTRICAL energy • Converts _______ SPONTANEOUS REDOX REACTION by the use of a ________________ ELECTRONS • _________ move from the metal that is OXIDIZED REDUCED ______ to the metal that is _______ ANODE CATHODE • Flow of electrons: ________________

II. Spontaneous Reactions on Voltaic Cells • • Voltaic Cell - a redox reaction whose two half-reactions are carried out separately, and the electrons given off by the oxidation half-reaction are used to power a device, and then given to the reduction half-reaction. ALSO CALLED A BATTERY (9 v) OR CELL (AAA, C, D) Voltaic Cell Animation and another set of animations: Complete Cell, Anode, Cathode, Salt Bridge

II. Spontaneous Reactions on Voltaic Cells • Parts of a Voltaic Wet Cell: • Electrode: A PIECE OF METAL WHERE OX. OR RED. OCCURS • Anode: ELECTRODE WHERE OXIDITION OCCURS (SIZE ↓ ) • Cathode: ELECTRODE WHERE REDUCTION OCCURS (SIZE ↑ ) • REMEMBER: “RED CAT and AN OX” REDUCTION OCCURS ON CATHODE ANODE HAS OXIDATION

II. Spontaneous Reactions on Voltaic Cells • Salt Bridge: + ions from bridge move into cathode half cell -- ions from bridge move into anode half cell Ions from the salt bridge flow to the solutions completing the circuit Keeps the overall charge neutral in the half cells • External Circuit: WIRE THAT CONDUCTS THE e-

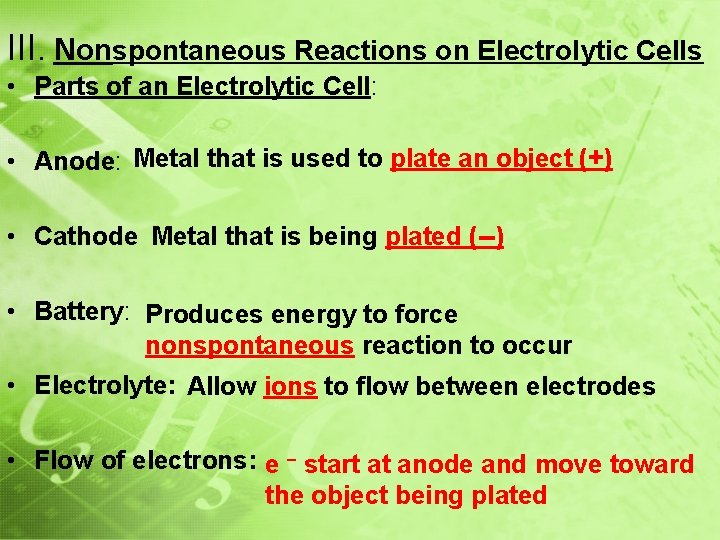
III. Nonspontaneous Reactions on Electrolytic Cells • Electrolytic Cell Animation

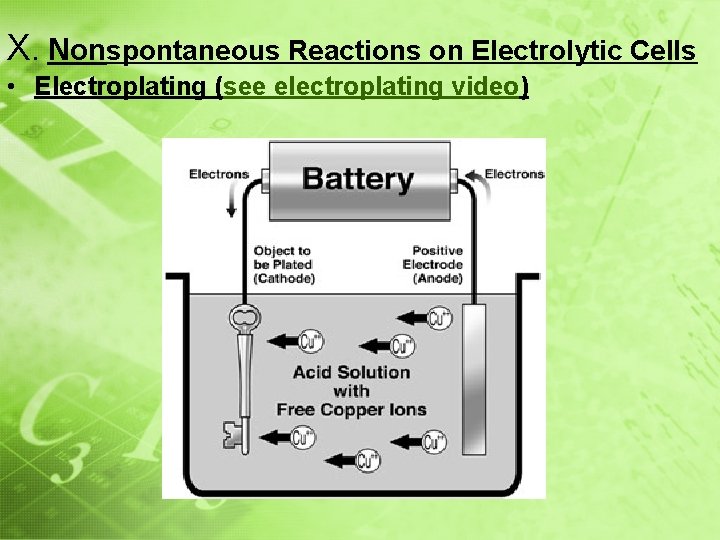
III. Nonspontaneous Reactions on Electrolytic Cells • Parts of an Electrolytic Cell: • Anode: Metal that is used to plate an object (+) • Cathode Metal that is being plated (--) • Battery: Produces energy to force nonspontaneous reaction to occur • Electrolyte: Allow ions to flow between electrodes • Flow of electrons: e – start at anode and move toward the object being plated

III. Nonspontaneous Reactions on Electrolytic Cells • Parts of a Electrolytic Cell: nonspontaneous redox reaction (the • Uses a ________ will NOT reaction _______ occur on its own) electricity force a chemical reaction to • Uses ______ to__________ occur electrolysis • This process is called ____________ • Used for: 1) electroplating 2) Isolation of an element in a compound (Na. Cl Na+Cl 2) 3) Purification of an element

X. Nonspontaneous Reactions on Electrolytic Cells • Electroplating (see electroplating video)

III. Nonspontaneous Reactions on Electrolytic Cells • The Car Battery (or rechargeable batteries) use both types of cells • Spontaneous Reaction to start the car • Nonspontaneous Reaction to charge the battery VIDEO

IV. Similarities and Differences Between Voltaic and Electrolytic Cells • Similarities • “AN OX and RED CAT” • e – flow from anode to cathode • (+) ions flow toward the cathode, (--) ions flow toward the anode • Anode gets smaller, cathode gets larger

V. Differences Between Voltaic and Electrolytic Cells Voltaic Electrolytic requires a battery is an electric cell _____________________________ negative Anode is __________ positive Cathode is ____________________ positive negative USES PRODUCES __________ electricity SPONTANEOUS REACTION NONSPONTANEOUS __________________ REACTION ½ Reactions are for… Two different elements (two different solutions) ½ Reactions are for… The same element (one solution both electrodes)