OXIDATION NUMBERS To determine whether a reaction is

OXIDATION NUMBERS • To determine whether a reaction is redox or not, we can assign oxidation numbers to each atom in the equation. If there is a change in oxidation number, there is a redox reaction occurring. Oxidation = increase in oxidation number Reduction = decrease in oxidation number

HALF-EQUATIONS • A redox equation can be separated into two equations to clearly show where the electrons are lost and gained. – One equation shows the oxidation process. – The other equation shows the reduction process. These two equations are called redox half-equations All redox equations are made up of two half-equations – in one equation the electrons are lost (oxidation) and in the other equation those electrons are gained (reduction).
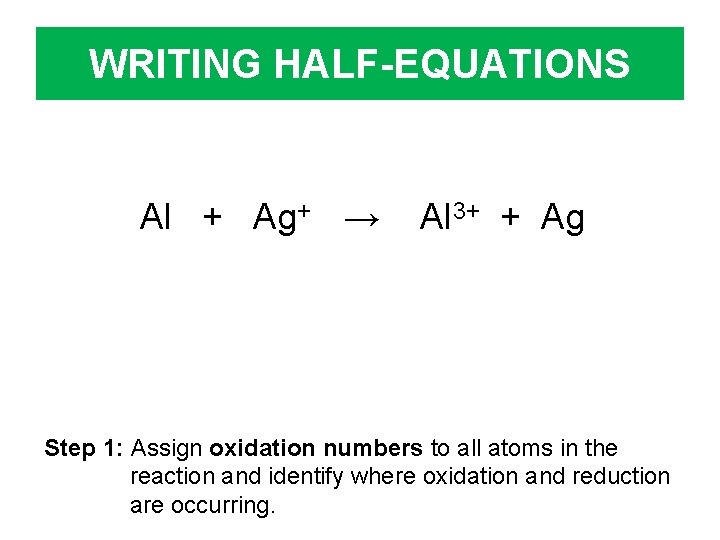
WRITING HALF-EQUATIONS Al + Ag+ → Al 3+ + Ag Step 1: Assign oxidation numbers to all atoms in the reaction and identify where oxidation and reduction are occurring.
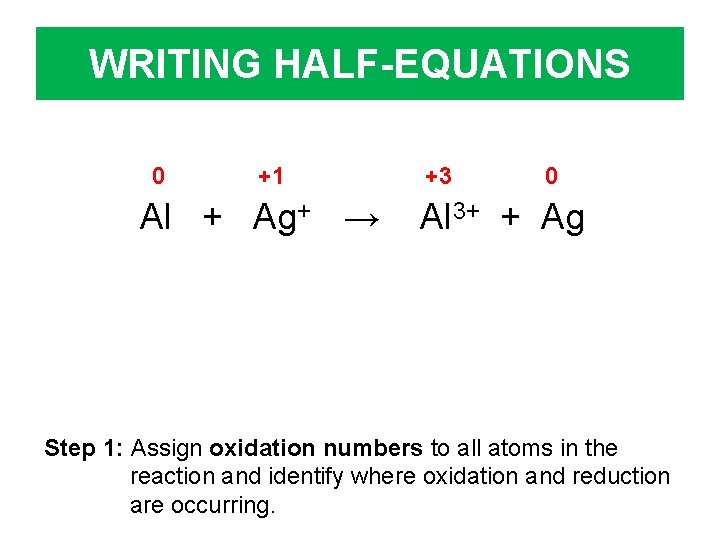
WRITING HALF-EQUATIONS 0 +1 Al + Ag+ → +3 0 Al 3+ + Ag Step 1: Assign oxidation numbers to all atoms in the reaction and identify where oxidation and reduction are occurring.
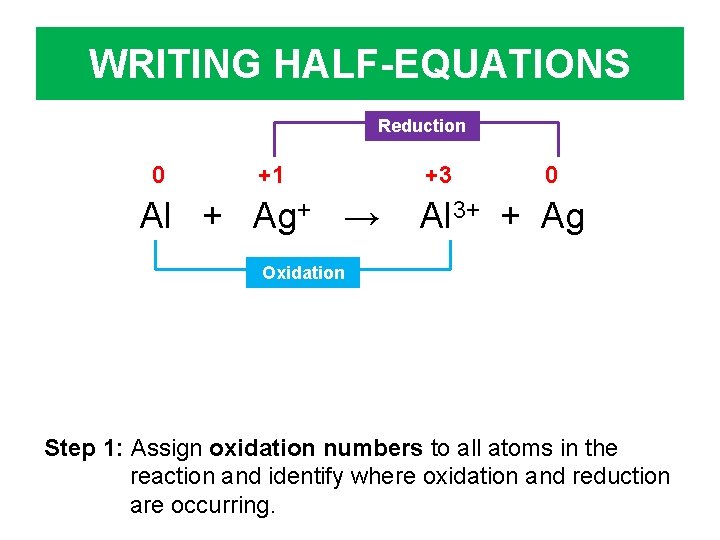
WRITING HALF-EQUATIONS Reduction 0 +1 Al + Ag+ → +3 0 Al 3+ + Ag Oxidation Step 1: Assign oxidation numbers to all atoms in the reaction and identify where oxidation and reduction are occurring.
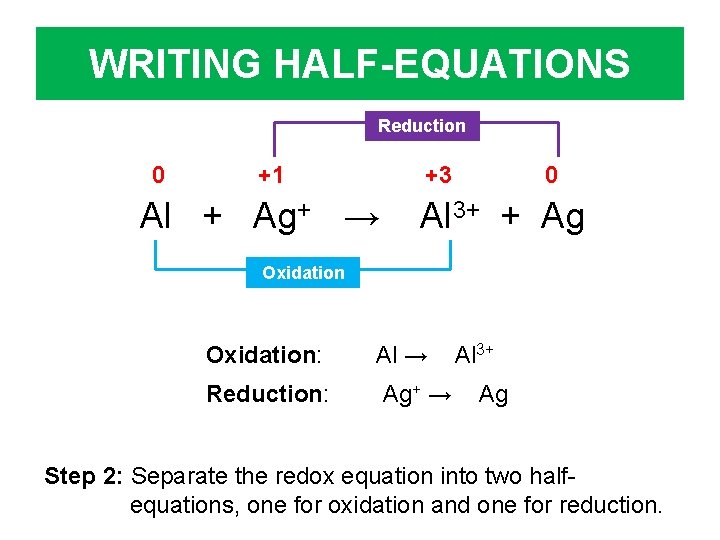
WRITING HALF-EQUATIONS Reduction 0 +1 +3 Al + Ag+ → 0 Al 3+ + Ag Oxidation: Al → Reduction: Ag+ → Al 3+ Ag Step 2: Separate the redox equation into two halfequations, one for oxidation and one for reduction.

WRITING HALF-EQUATIONS • Half reactions must always be balanced. They must have the same number and type of atoms, and the same charge, on each side of the equation. – Note: balancing a half-reaction is done slightly differently to balancing normal chemical equations. • To balance a redox half-equation, we use the “A O H C” method: Balance Atoms other than O or H Balance Oxygens by adding H 2 O Balance Hydrogens by adding H+ Balance Charge by adding e-
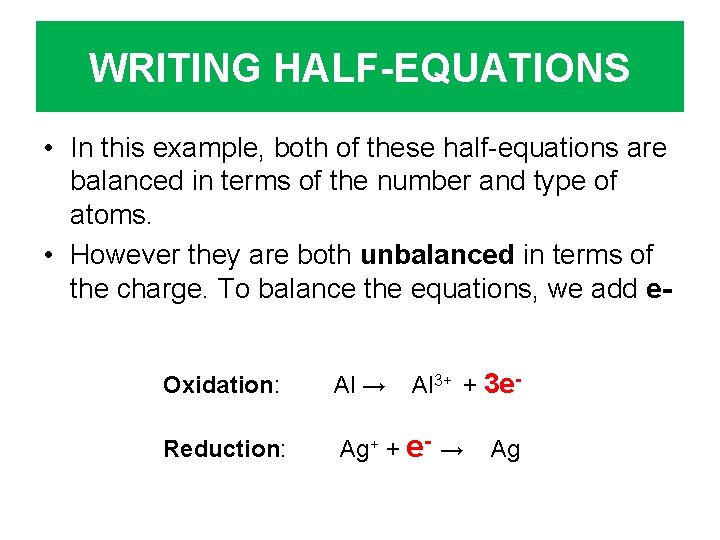
WRITING HALF-EQUATIONS • In this example, both of these half-equations are balanced in terms of the number and type of atoms. • However they are both unbalanced in terms of the charge. To balance the equations, we add e. Al 3+ + 3 e- Oxidation: Al → Reduction: Ag+ + e- → Ag
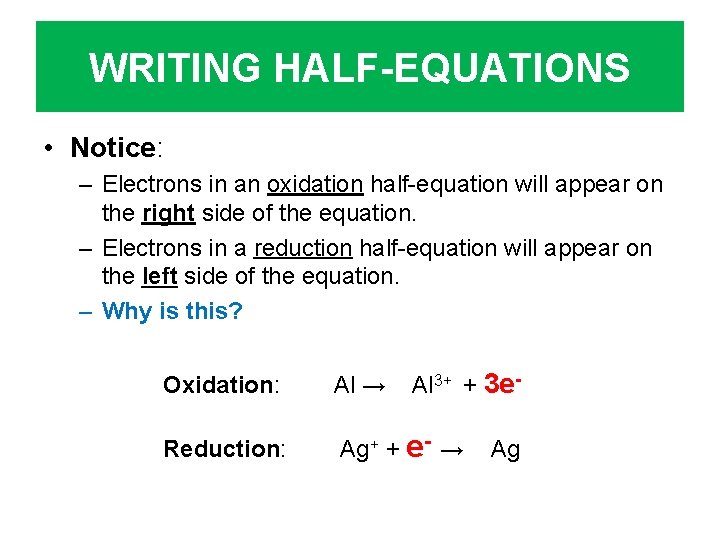
WRITING HALF-EQUATIONS • Notice: – Electrons in an oxidation half-equation will appear on the right side of the equation. – Electrons in a reduction half-equation will appear on the left side of the equation. – Why is this? Al 3+ + 3 e- Oxidation: Al → Reduction: Ag+ + e- → Ag
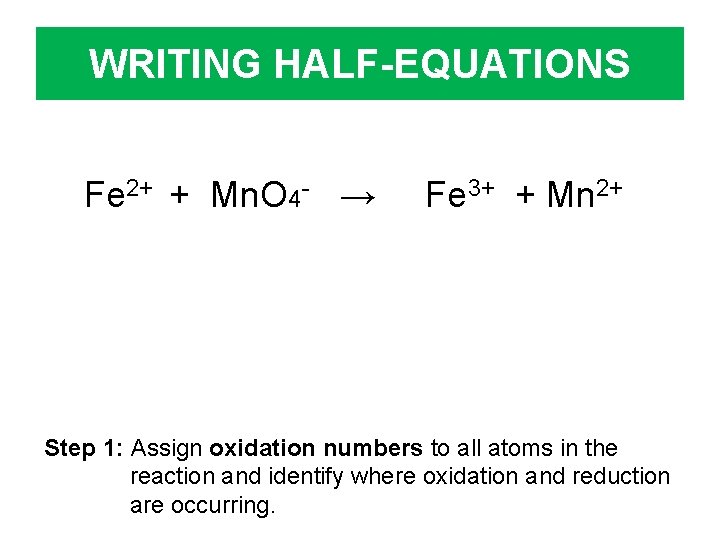
WRITING HALF-EQUATIONS Fe 2+ + Mn. O 4 - → Fe 3+ + Mn 2+ Step 1: Assign oxidation numbers to all atoms in the reaction and identify where oxidation and reduction are occurring.
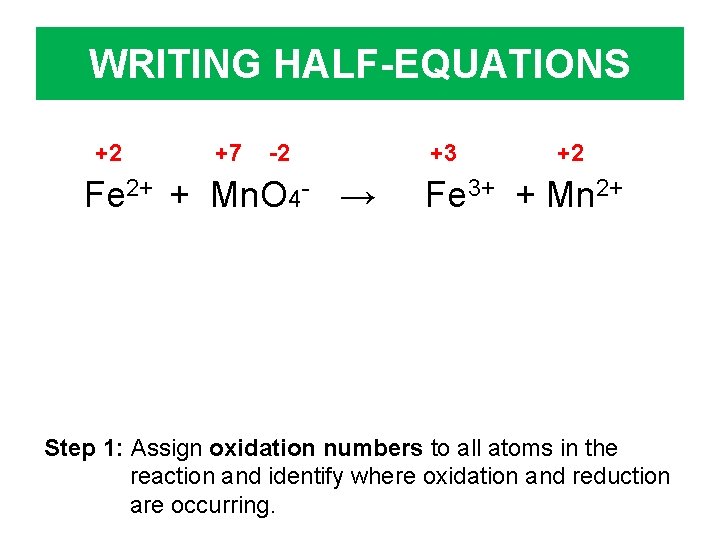
WRITING HALF-EQUATIONS +2 +7 -2 Fe 2+ + Mn. O 4 - → +3 +2 Fe 3+ + Mn 2+ Step 1: Assign oxidation numbers to all atoms in the reaction and identify where oxidation and reduction are occurring.
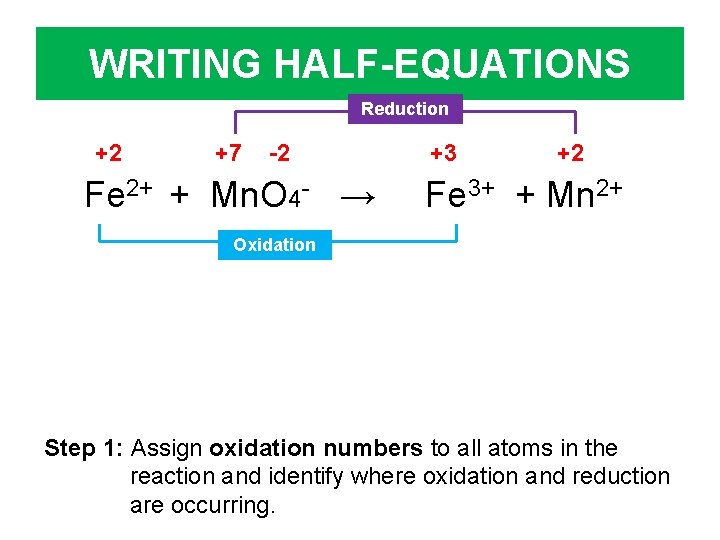
WRITING HALF-EQUATIONS Reduction +2 +7 -2 Fe 2+ + Mn. O 4 - → +3 +2 Fe 3+ + Mn 2+ Oxidation Step 1: Assign oxidation numbers to all atoms in the reaction and identify where oxidation and reduction are occurring.
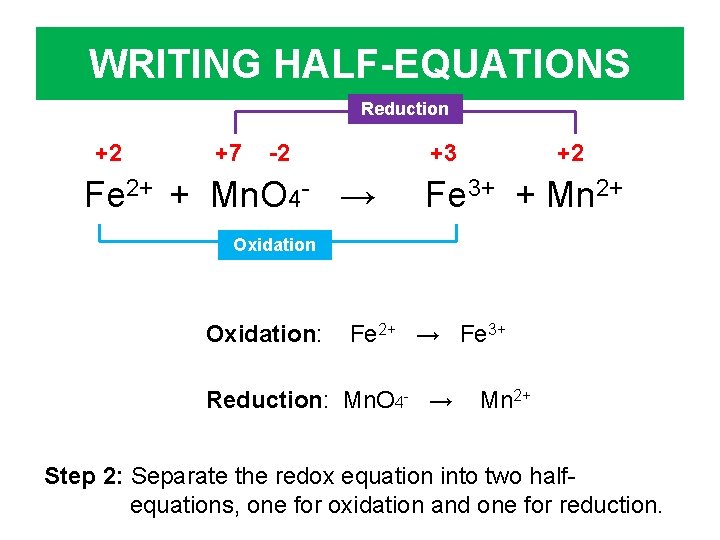
WRITING HALF-EQUATIONS Reduction +2 +7 -2 +3 Fe 2+ + Mn. O 4 - → +2 Fe 3+ + Mn 2+ Oxidation: Fe 2+ → Fe 3+ Reduction: Mn. O 4 - → Mn 2+ Step 2: Separate the redox equation into two halfequations, one for oxidation and one for reduction.
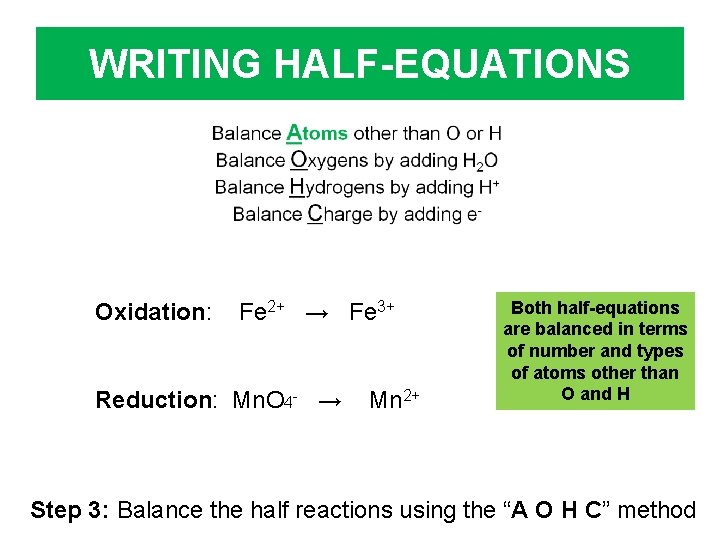
WRITING HALF-EQUATIONS Oxidation: Fe 2+ → Fe 3+ Reduction: Mn. O 4 - → Mn 2+ Both half-equations are balanced in terms of number and types of atoms other than O and H Step 3: Balance the half reactions using the “A O H C” method
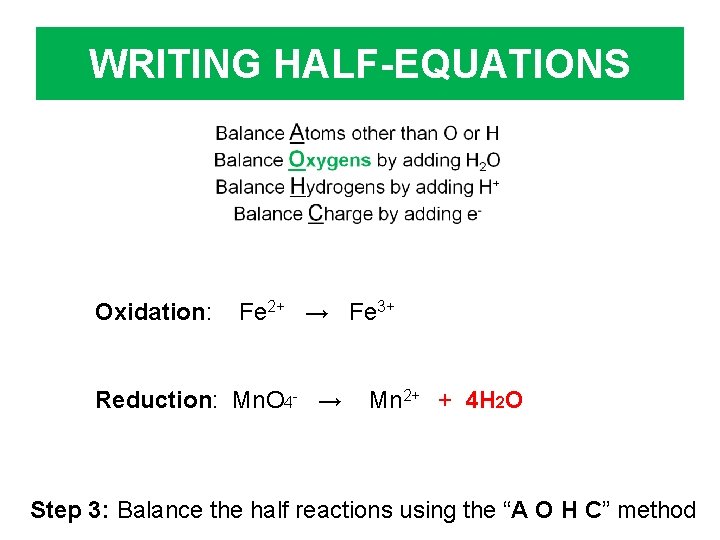
WRITING HALF-EQUATIONS Oxidation: Fe 2+ → Fe 3+ Reduction: Mn. O 4 - → Mn 2+ + 4 H 2 O Step 3: Balance the half reactions using the “A O H C” method
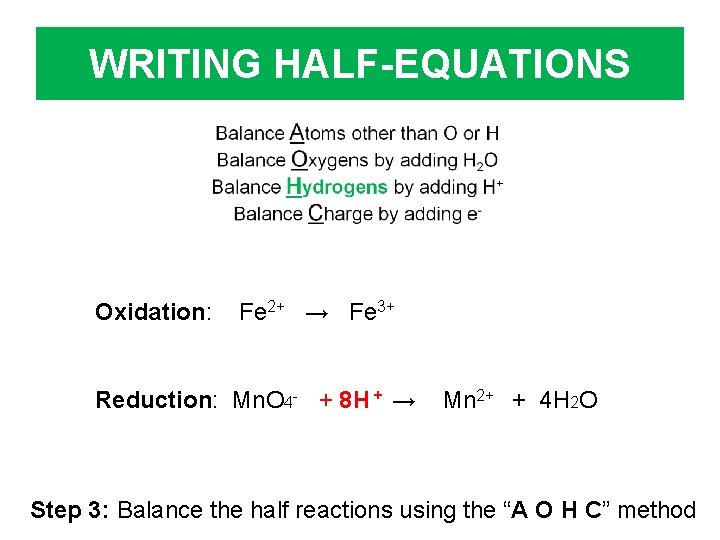
WRITING HALF-EQUATIONS Oxidation: Fe 2+ → Fe 3+ Reduction: Mn. O 4 - + 8 H + → Mn 2+ + 4 H 2 O Step 3: Balance the half reactions using the “A O H C” method
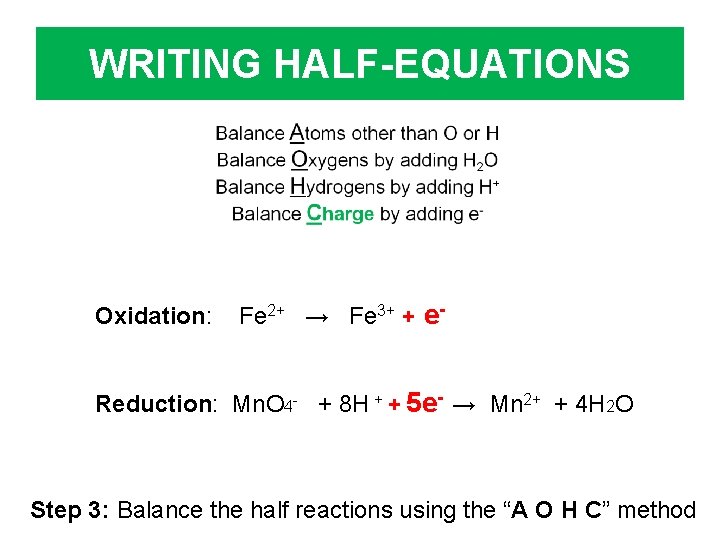
WRITING HALF-EQUATIONS Oxidation: Fe 2+ → Fe 3+ + e- Reduction: Mn. O 4 - + 8 H + + 5 e- → Mn 2+ + 4 H 2 O Step 3: Balance the half reactions using the “A O H C” method
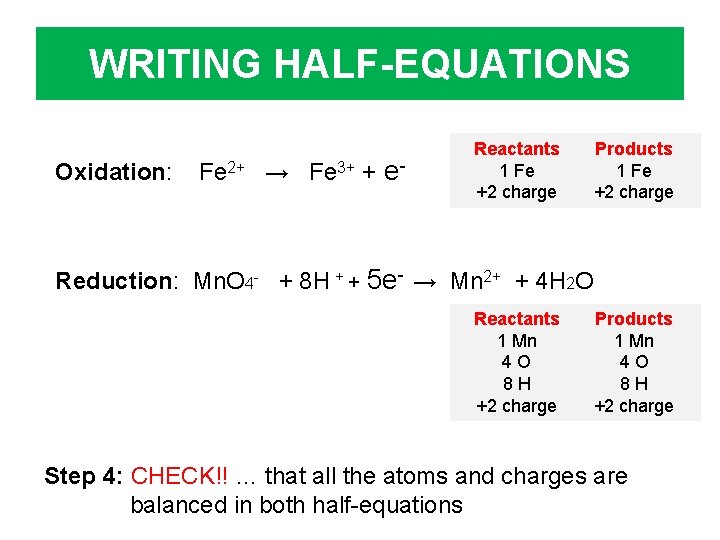
WRITING HALF-EQUATIONS Oxidation: Fe 2+ → Fe 3+ + e- Reactants 1 Fe +2 charge Products 1 Fe +2 charge Reduction: Mn. O 4 - + 8 H + + 5 e- → Mn 2+ + 4 H 2 O Reactants 1 Mn 4 O 8 H +2 charge Products 1 Mn 4 O 8 H +2 charge Step 4: CHECK!! … that all the atoms and charges are balanced in both half-equations
- Slides: 18