Oxidation Numbers The Myth and Meaning Oxidation Numbers

Oxidation Numbers The Myth and Meaning!

Oxidation Numbers Number used to indicate distribution of electrons No specific physical meaning Indicates what electrons of atom are doing during bond formation Assigned with rules!

The RULES Pure elements have oxidation number zero More electronegative assigned oxidation number it would have if it were an anion Fluorine has oxidation number of -1 Oxygen has oxidation number of -2 unless it is in a peroxide (-1) or bonded with a halogen (+2) Hydrogen has oxidation number of +1 unless bonded with metals (-1) Sum of oxidation numbers In a compound is zero
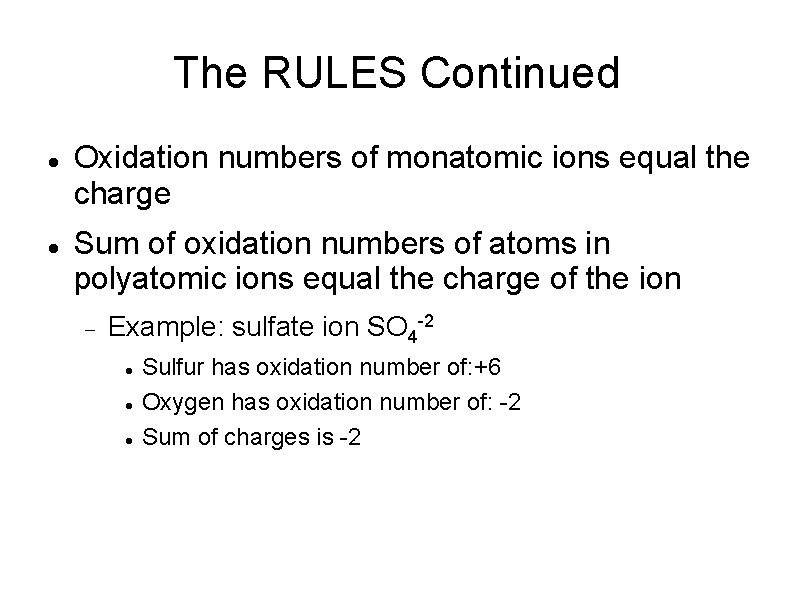
The RULES Continued Oxidation numbers of monatomic ions equal the charge Sum of oxidation numbers of atoms in polyatomic ions equal the charge of the ion Example: sulfate ion SO 4 -2 Sulfur has oxidation number of: +6 Oxygen has oxidation number of: -2 Sum of charges is -2
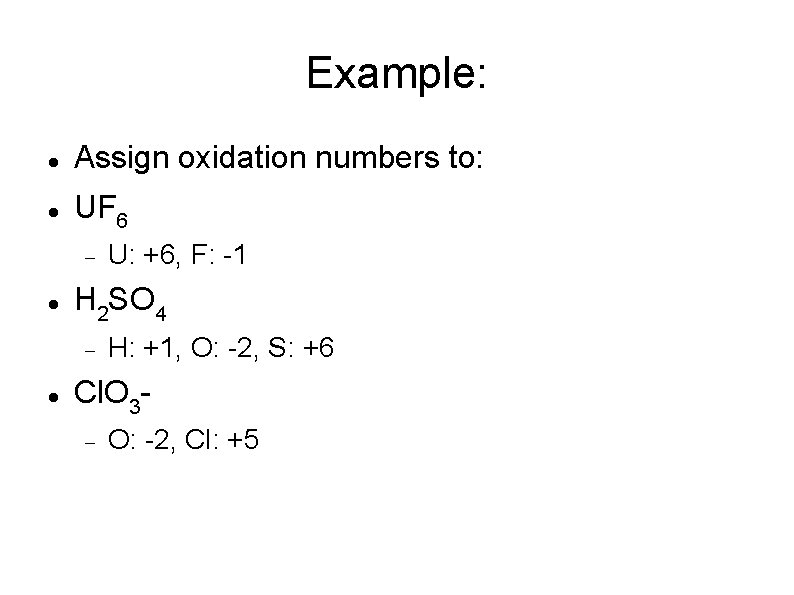
Example: Assign oxidation numbers to: UF 6 H 2 SO 4 U: +6, F: -1 H: +1, O: -2, S: +6 Cl. O 3 O: -2, Cl: +5
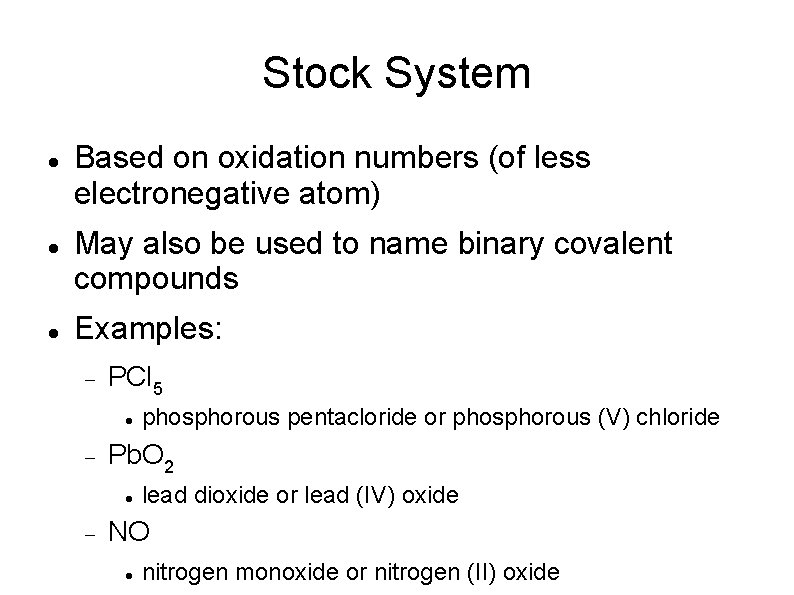
Stock System Based on oxidation numbers (of less electronegative atom) May also be used to name binary covalent compounds Examples: PCl 5 Pb. O 2 phosphorous pentacloride or phosphorous (V) chloride lead dioxide or lead (IV) oxide NO nitrogen monoxide or nitrogen (II) oxide
- Slides: 6