Oxidation Numbers OxidationReduction Mr Shields Regents Chemistry U

Oxidation Numbers & Oxidation-Reduction Mr. Shields Regents Chemistry U 14 L 01 1

Oxidation Numbers We’ve talked about oxidation nos. before. What is the Definition of an atoms oxidation number? The number of electrons an atom tends to give up or gain in a Chemical reaction Where can we find an elements oxidation number? What are the oxidation nos. for O and S in the compound SO 2 ? Looking at the reference table what is oxygen’s oxidation no. If Oxygen is -2 Sulfur must be +4 (Why? ) 2

Oxidation Numbers But what if we had looked up the oxidation no. of Sulfur first? The table say’s sulfurs most common oxidation no. is -2 so Oxygen would be +1 (why). But that can’t be. There is no +1 for oxygen. So how do we figure this out? There a few RULES we need to learn when assigning Oxidation Numbers to atoms in compounds. 3

Ox. Number Rules Rule 1: Rule 2: Rule 3: Rule 4: Rule 5: Rule 6: Rule 7: Rule 8: Uncombined atoms are always 0 Group 1 atoms are always Group 2 atoms are always Oxygen is always exception: Peroxides like H 2 O 2, Na 2 O 2 oxygen is In OF 2 oxygen is (know these) Fluorine is always The other Halogens are “usually” Hydrogen combined with non-metals +1 example: HBr Hydrogen combined with metals (hydrides) -1 example: Ca. H 2 +1 +2 -2 -1 +2 -1 -1 Rule 9: Total of the charges for all atoms in a compound is zero (0) Rule 10: Total of the charges for all atoms in a polyatomic is equal to the charge of the polyatomic ion 4

Oxidation Numbers Example: What is the oxidation number of each element in the following compound. Sr. Cl 2 Sr is group 2 so it always has an oxidation no. of +2 The sum of all the charges of a compound is zero so the 2 Cl must be -2. Therefore each of the Cl has an Ox. Number of -1 5

Oxidation Numbers Example: What is the oxidation number of each element in the following compound. Pb. Cr. O 4 First you need to recognize that Cr. O 4 is a polyatomic with a charge of -2 Since Oxygen is always -2, Cr must be +6 (why? ) Since Cr. O 4 has a charge of -2, Pb must be +2 6

Oxidation Numbers Problem: What is the oxidation number of each element in NH 4 NO 3 In this compound there are 2 polyatomics (NH 4+, NO 3 -) NH 4+: H +1 N -3 (Always) why? NO 3 -: O -2 N +5 (Always) why? 7

Find the oxidation #’s for each element in the compound H 2 S 2 O 7 H is always +1 when bonded to a non-metal O is always -2 (except in H 2 O 2 and OF 2) To find Sulfur, the sum of charges must equal 0 2 (+1) + 2 X + 7 (-2) = 0 2 + 2 X + -14 = 0 2 X - 12 =0 2 X = 12 X = 6 Sulfur = +6 8

Redox We’re now going to discuss a topic that involves something called Oxidation & Reduction - These reactions are commonly known as REDOX REACTIONS SO … what is oxidation and what is reduction? Originally oxidation referred to any reaction in which Oxygen was one of the reactants. Ex: 4 Fe + 3 O 2 2 Fe 2 O 3 2 Cu + O 2 2 Cu. O CH 4 + 2 O 2 CO 2 + 2 H 2 O 9
Redox Opposite of Oxidation is Reduction. Originally, this referred to any reaction in which Oxygen was removed from a reactant. Ex: 2 Fe 2 O 3 + 3 C 4 Fe + 3 CO 2 Cu. O + H 2 Cu + H 2 O Today, oxidation & Reduction still refer to these type of reactions but both have taken on a much broader meaning Recall that Oxygen is a very electronegative element - EN = 3. 5 on a scale of 0 - 4 - This means it very strongly draws electrons to itself (how many electrons does it want ? ) Right… 2 e- : O + 2 e- O-2 10

Redox Since Oxygen wants electrons, substances that react with oxygen Have to lose electrons - Cu: + O Cu+2 O-2 From this comes the broader definition of Oxidation/Reduction Oxidation is the process by which substances lose 1 or more e. Reduction is the process by which substances gain 1 or more e. HOW CAN WE REMEMBER THIS? - Remember the phrase OIL RIG Oxidation Is Lose of electrons / Reduction Is Gain of electrons 11

Redox NOTE: Oxidation and reduction ALWAYS occur together Electrons can’t just be lost they need to be accepted by some Other substance. Metals are typically oxidized (lose electrons) Na Na+ +1 e- Non-metals are typically reduced (gain electrons) Cl + 1 e- Cl 12

Half-cell reactions The Equation that show an element either gains or loses Electrons is called a HALF CELL reaction For example what is oxidized and what is reduced in the following equation and what are the 2 half cell reactions? Fe + S Fe. S sulfur is reduced (why? ) S 0 + 2 e- S-2 This is known as the REDUCTION half cell iron is oxidized (why? ) Fe 0 Fe+2 + 2 e. This is known as the OXIDATION half cell 13

Half cell rxns Oxidation half cell Rxns lose electrons (they shows up on the product side) 2 F- F 2 (g) + 2 e Oxidation Mn+2 (s) Mn+7 + 5 e – Oxidation Reduction half cell Rxns gain electrons (they show up on the reactant side) F 2(g) + 2 e - 2 FReduction Mn+7 + 5 e - Mn+2 (s) Reduction 14
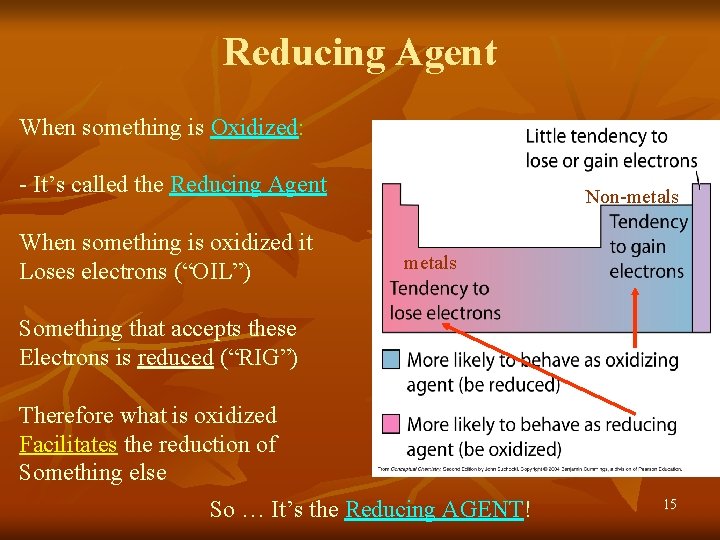
Reducing Agent When something is Oxidized: - It’s called the Reducing Agent When something is oxidized it Loses electrons (“OIL”) Non-metals Something that accepts these Electrons is reduced (“RIG”) Therefore what is oxidized Facilitates the reduction of Something else So … It’s the Reducing AGENT! 15

Reducing agent Let’s look at an example of a reducing agent at work Fe + O 2 Fe 2 O 3 What is the Reducing Agent? And what is Reduced? The first question to ask is which is “WHAT’S OXIDIZED? ” Iron is losing electrons: Fe 0 Fe+3 so Fe is oxidized. But Fe has to give it’s electrons to something else. What is it? Oxygen. So O 2 is reduced. Therefore, Fe is the Reducing Agent 16

Oxidizing Agent We said that when something is Oxidized it’s the Reducing Agent. So… When something is Reduced It’s called the Oxidizing Agent. We know that when something GAINS electrons it’s reduced Those electrons must come from another Atom or Ion. The atom or ion that lost those electrons is therefore oxidized. For example: 2 H 2 + O 2 H 20 Oxidation half cell: Reduction half cell: (what is the Oxidizing agent? ) H 0 H+1 + 1 e. O 0 + 2 e- O-2 Since Oxygen is reduced; it’s the “agent” that oxidizes Hydrogen, so it’s the Oxidizing agent. 17

Problem: for the following Reaction C(s) + Cl 2(g) CCl 4 (l) A) B) C) D) What is reduced? What gets oxidized? What is the reducing agent? What is the oxidizing agent? Cl 0 C 0 Cl 0 Problem: for the following Reaction 2 Mg. O 2 Mg + O 2 Mg+2 A) What is reduced? O-2 B) What gets oxidized? O-2 C) What is the reducing agent? Mg+2 D) What is the oxidizing agent? E) Write the Oxidation and Reduction Half cell reactions. 18
- Slides: 18