Oxidation Numbers LESSON 2 TEST 9 1 PART

Oxidation Numbers LESSON 2 TEST 9. 1 PART 2

Oxidation Numbers • actual or hypothetical charges assigned using a set of rules • used to describe REDOX reactions with covalent reactants or products • used to identify REDOX rxn’s; identify oxidizing and reducing agents
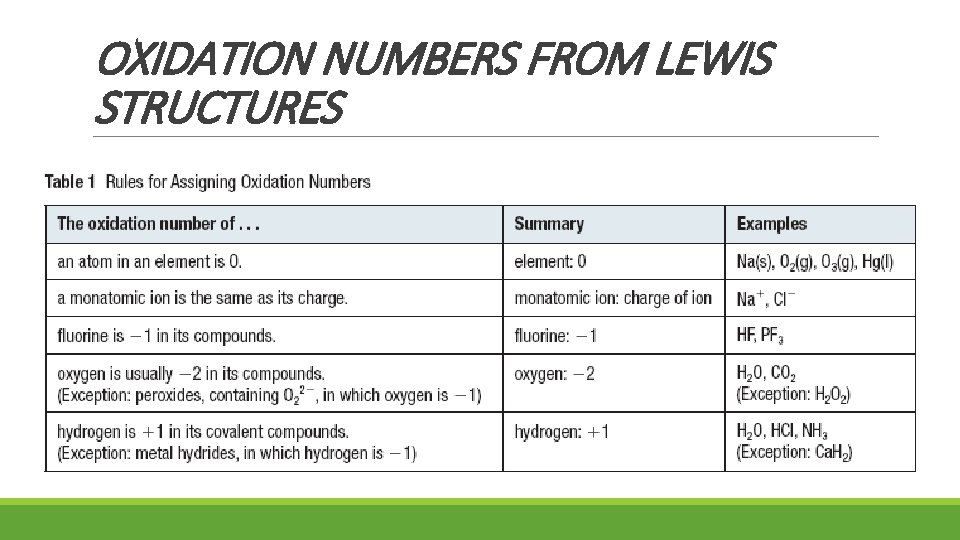
OXIDATION NUMBERS FROM LEWIS STRUCTURES
OXIDATION NUMBERS FROM LEWIS STRUCTURES • consider all bonding electrons to be “owned” by the more electronegative atom • if electronegativity is the same (Cl 2) then the bond is non-polar covalent, therefore, consider each atom to “own” one of the shared electrons • NOTE: • the +/- sign is written before the number (O-2) in an oxidation number • the +/- sign is written after the number (O 2 -) in an ionic charge
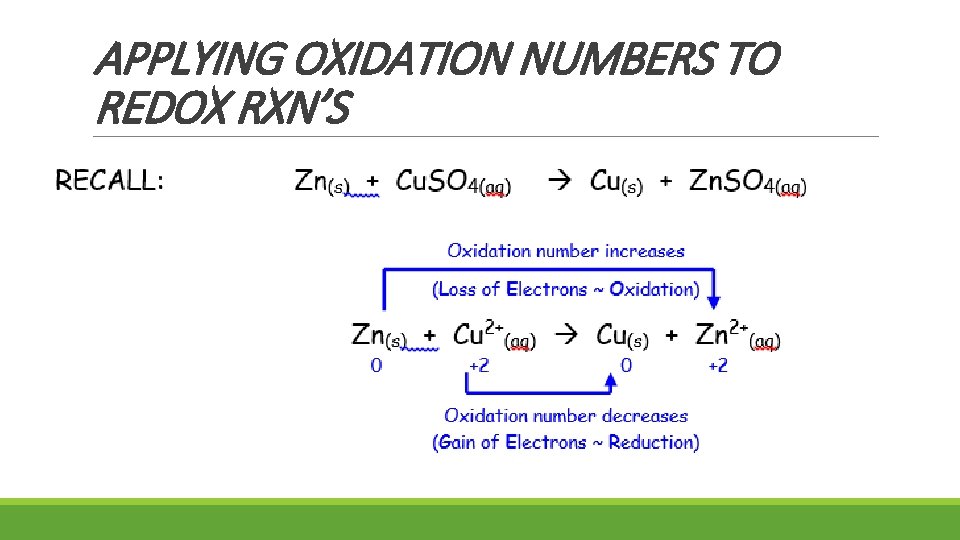
APPLYING OXIDATION NUMBERS TO REDOX RXN’S
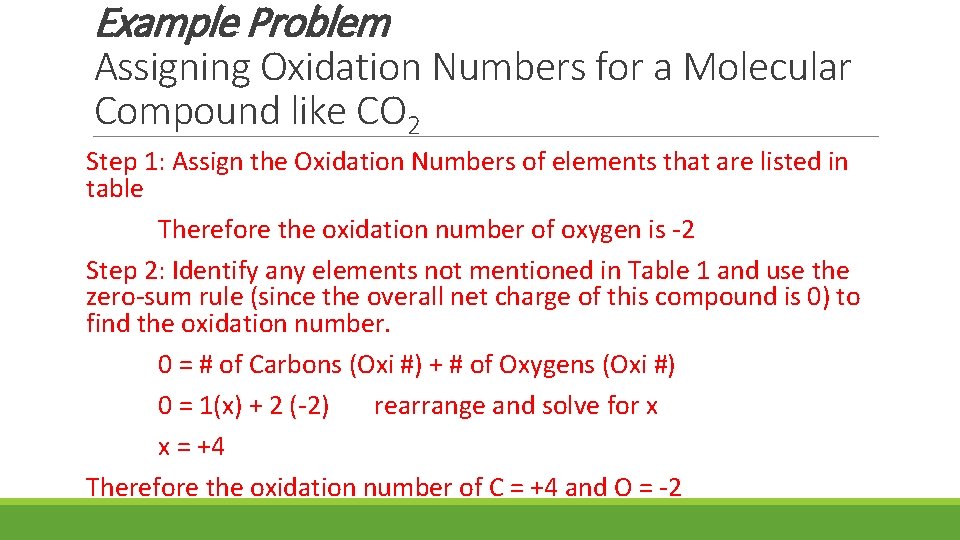
Example Problem Assigning Oxidation Numbers for a Molecular Compound like CO 2 Step 1: Assign the Oxidation Numbers of elements that are listed in table Therefore the oxidation number of oxygen is -2 Step 2: Identify any elements not mentioned in Table 1 and use the zero-sum rule (since the overall net charge of this compound is 0) to find the oxidation number. 0 = # of Carbons (Oxi #) + # of Oxygens (Oxi #) 0 = 1(x) + 2 (-2) rearrange and solve for x x = +4 Therefore the oxidation number of C = +4 and O = -2
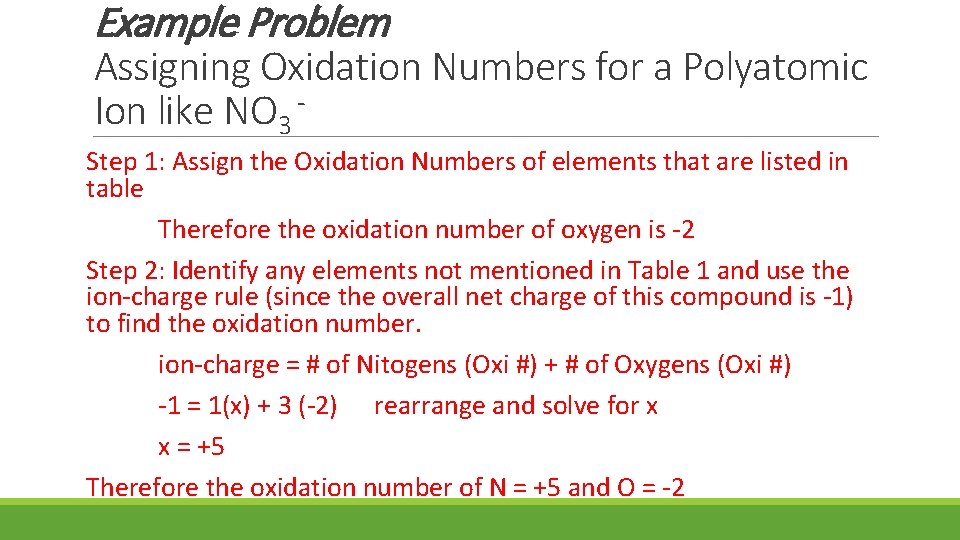
Example Problem Assigning Oxidation Numbers for a Polyatomic Ion like NO 3 Step 1: Assign the Oxidation Numbers of elements that are listed in table Therefore the oxidation number of oxygen is -2 Step 2: Identify any elements not mentioned in Table 1 and use the ion-charge rule (since the overall net charge of this compound is -1) to find the oxidation number. ion-charge = # of Nitogens (Oxi #) + # of Oxygens (Oxi #) -1 = 1(x) + 3 (-2) rearrange and solve for x x = +5 Therefore the oxidation number of N = +5 and O = -2
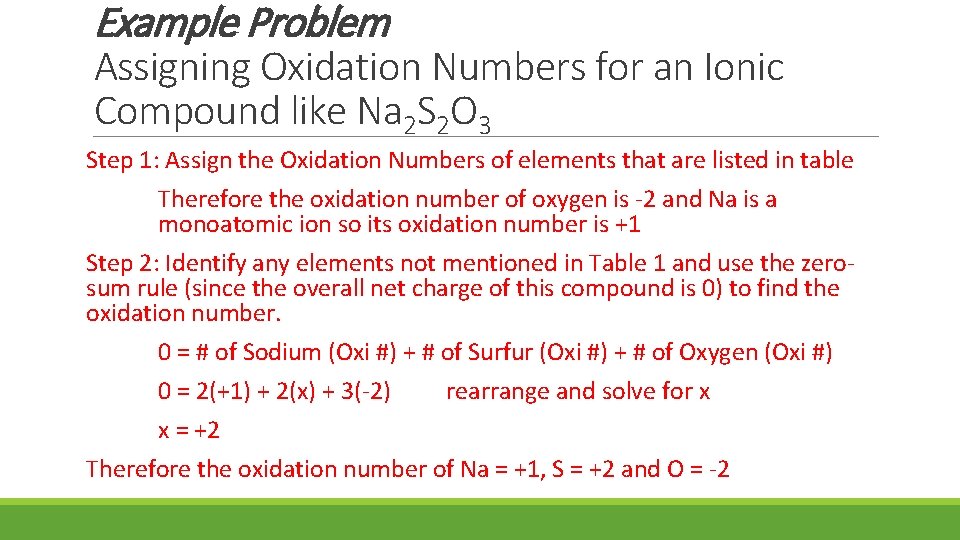
Example Problem Assigning Oxidation Numbers for an Ionic Compound like Na 2 S 2 O 3 Step 1: Assign the Oxidation Numbers of elements that are listed in table Therefore the oxidation number of oxygen is -2 and Na is a monoatomic ion so its oxidation number is +1 Step 2: Identify any elements not mentioned in Table 1 and use the zerosum rule (since the overall net charge of this compound is 0) to find the oxidation number. 0 = # of Sodium (Oxi #) + # of Surfur (Oxi #) + # of Oxygen (Oxi #) 0 = 2(+1) + 2(x) + 3(-2) rearrange and solve for x x = +2 Therefore the oxidation number of Na = +1, S = +2 and O = -2
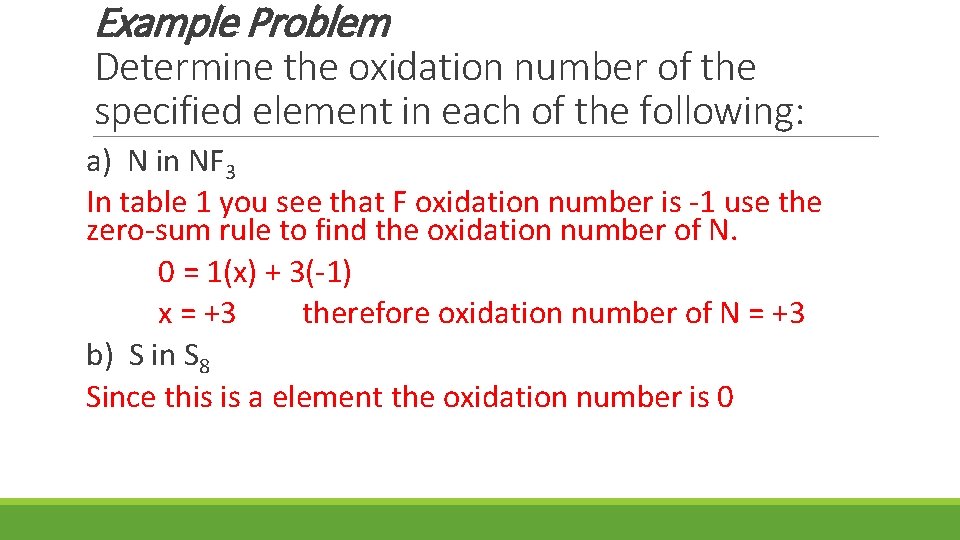
Example Problem Determine the oxidation number of the specified element in each of the following: a) N in NF 3 In table 1 you see that F oxidation number is -1 use the zero-sum rule to find the oxidation number of N. 0 = 1(x) + 3(-1) x = +3 therefore oxidation number of N = +3 b) S in S 8 Since this is a element the oxidation number is 0
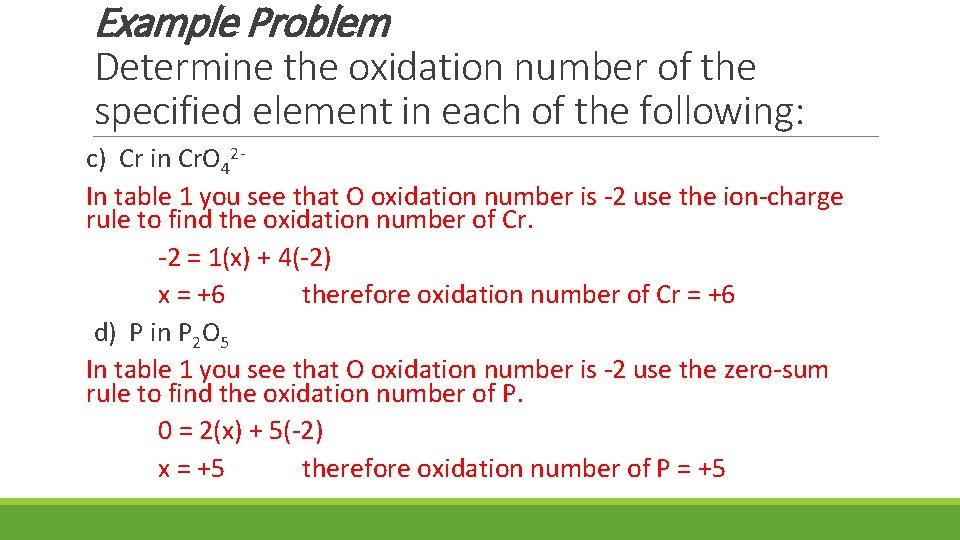
Example Problem Determine the oxidation number of the specified element in each of the following: c) Cr in Cr. O 42 In table 1 you see that O oxidation number is -2 use the ion-charge rule to find the oxidation number of Cr. -2 = 1(x) + 4(-2) x = +6 therefore oxidation number of Cr = +6 d) P in P 2 O 5 In table 1 you see that O oxidation number is -2 use the zero-sum rule to find the oxidation number of P. 0 = 2(x) + 5(-2) x = +5 therefore oxidation number of P = +5
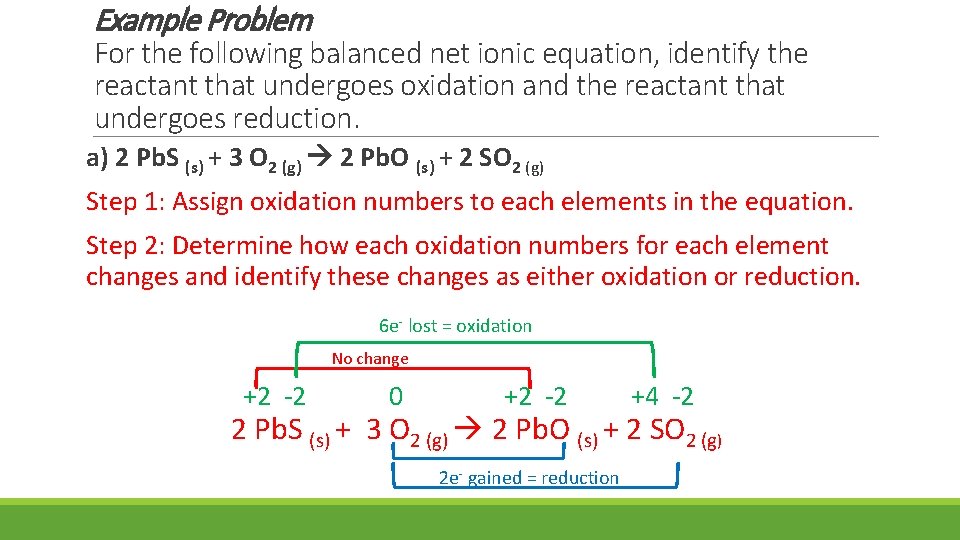
Example Problem For the following balanced net ionic equation, identify the reactant that undergoes oxidation and the reactant that undergoes reduction. a) 2 Pb. S (s) + 3 O 2 (g) 2 Pb. O (s) + 2 SO 2 (g) Step 1: Assign oxidation numbers to each elements in the equation. Step 2: Determine how each oxidation numbers for each element changes and identify these changes as either oxidation or reduction. 6 e- lost = oxidation No change +2 -2 0 +2 -2 +4 -2 2 Pb. S (s) + 3 O 2 (g) 2 Pb. O (s) + 2 SO 2 (g) 2 e- gained = reduction
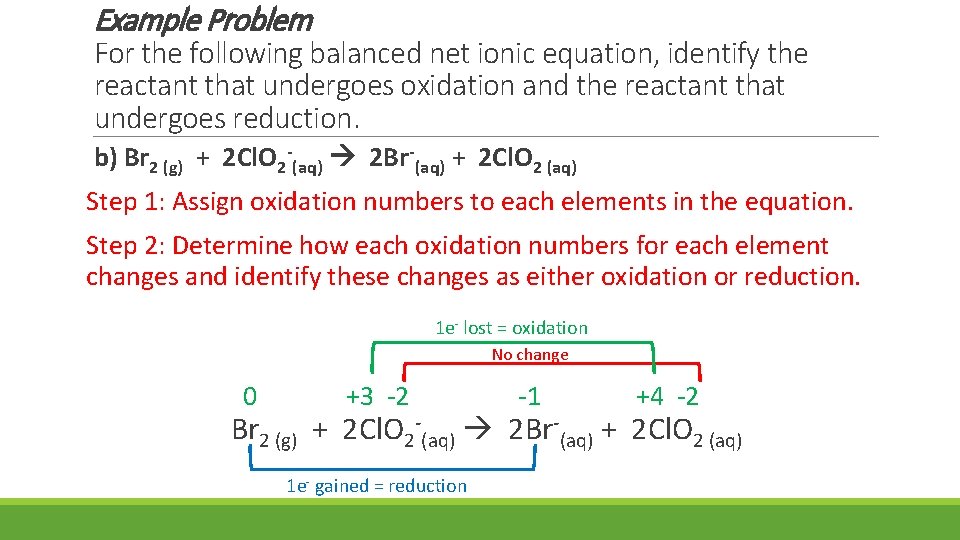
Example Problem For the following balanced net ionic equation, identify the reactant that undergoes oxidation and the reactant that undergoes reduction. b) Br 2 (g) + 2 Cl. O 2 -(aq) 2 Br-(aq) + 2 Cl. O 2 (aq) Step 1: Assign oxidation numbers to each elements in the equation. Step 2: Determine how each oxidation numbers for each element changes and identify these changes as either oxidation or reduction. 1 e- lost = oxidation No change 0 +3 -2 -1 +4 -2 Br 2 (g) + 2 Cl. O 2 -(aq) 2 Br-(aq) + 2 Cl. O 2 (aq) 1 e- gained = reduction

Homework 1) Work on the Oxidation Worksheet and hand it in 2) Answer Practice Problems for extra practice Pg. 604 #1 -4, Pg. 606 #1 -3, Pg. 607 #3 -10
- Slides: 13