OXIDATION AND REDUCTION REACTION REDOX 1 Types of


















- Slides: 57

OXIDATION AND REDUCTION REACTION REDOX 1

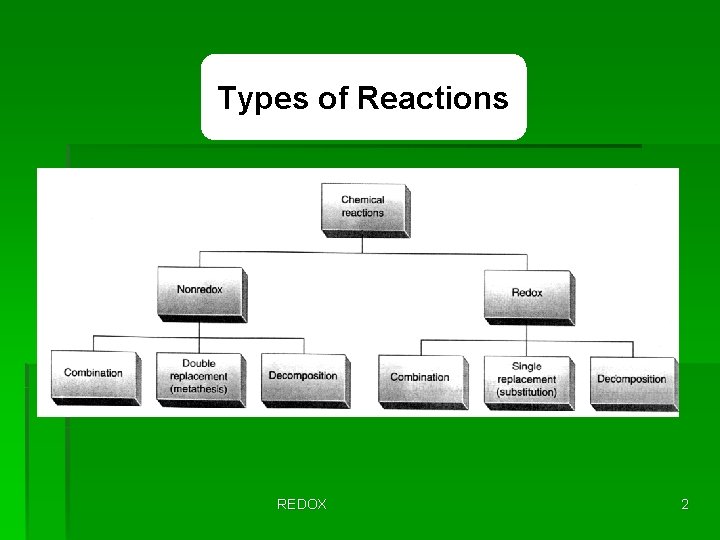
Types of Reactions REDOX 2

Redox Reaction § Is the reaction that involve the transfer of electrons from a reducing agent to an oxidizing agent REDOX 3

TYPE OF REDOX REACTION § OUT OF BODY REDOX § IN THE BODY 4

Electrochemistry § Is the branch of chemistry that deals with the interconversion of electrical energy and chemical energy § Electrochemical processes are redox reactions in which the energy released by a spontaneous reaction is converted to electricity or in which electrical energy is used to cause a nonspontaneous reaction to occur REDOX 5

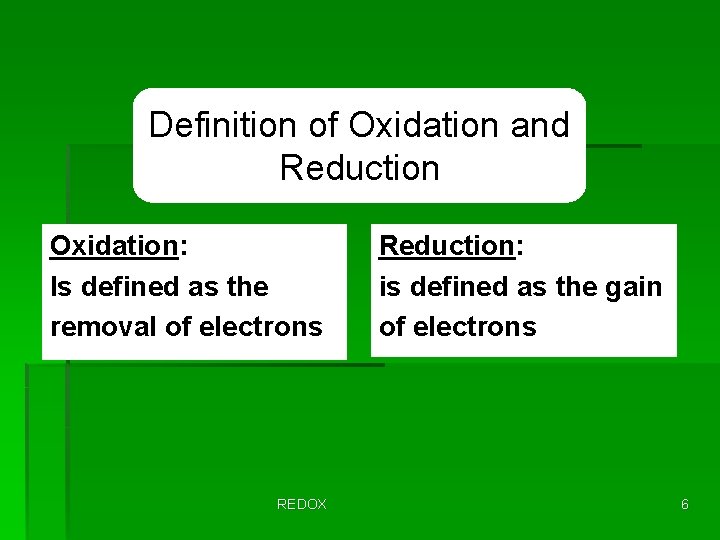
Definition of Oxidation and Reduction Oxidation: Is defined as the removal of electrons REDOX Reduction: is defined as the gain of electrons 6

Common uses of the terms oxidation and reduction Term Oxidation Meaning To Combine with oxygen To lose hydrogen To lose electron To increase in oxidation Reduction number To lose oxygen To combine with hydrogen To gain electron To decrease in oxidation REDOX number 7

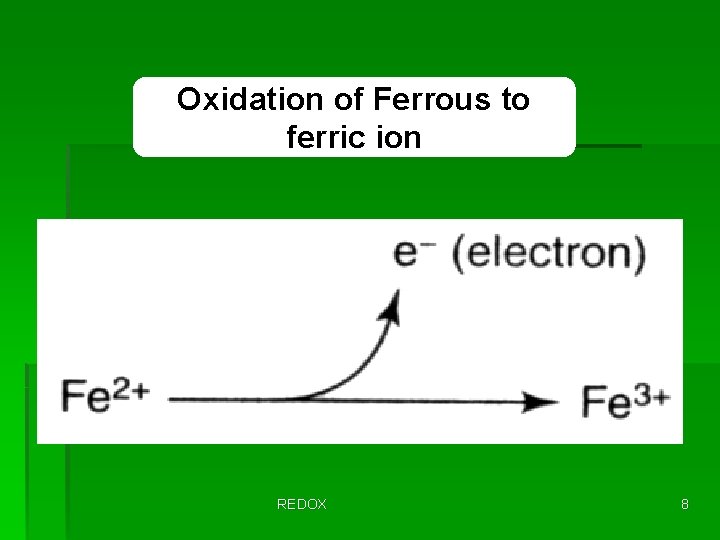
Oxidation of Ferrous to ferric ion REDOX 8

OXIDATION NUMBER § Oxidation numbers are the charges atoms in a compound would have if the electrons of each bond belonged to the more electronegative atoms. REDOX 9

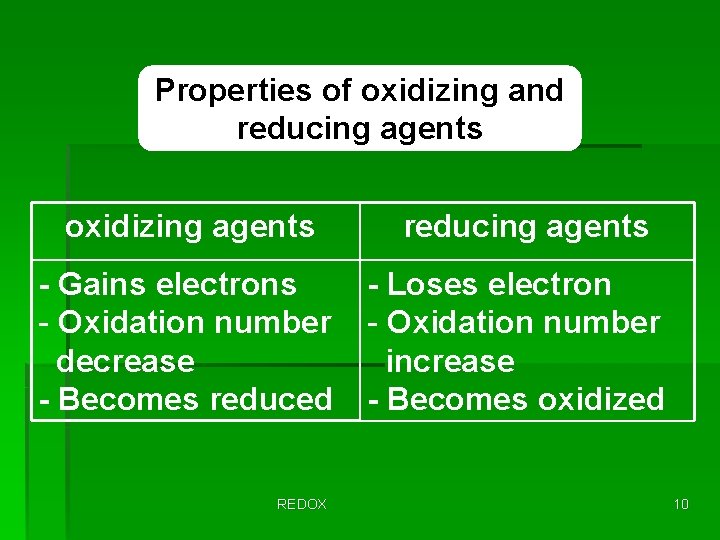
Properties of oxidizing and reducing agents oxidizing agents - Gains electrons - Oxidation number decrease - Becomes reduced REDOX reducing agents - Loses electron - Oxidation number increase - Becomes oxidized 10

THE RULES FOR ASSIGNING OXIDATION NUMBER 1. Atoms of any element not combined with atoms from another element have oxidation numbers of zero. Examples: The oxidation number of the atom in N 2, Br 2, Cl 2, P 4 and S 2 are zero REDOX continued 11

2. The oxidation numbers of monatomicions equal their ionic charge. Examples: The oxidation numbers of Na+ and K+ are +1, of Ca 2+, Cu 2+, and Mg 2+ are +2, and Cl¯ and Br¯ are – 1. REDOX continued 12

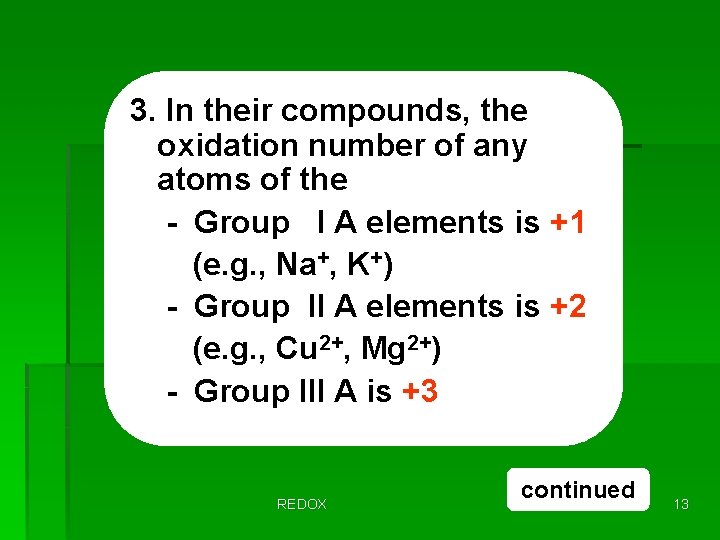
3. In their compounds, the oxidation number of any atoms of the - Group I A elements is +1 (e. g. , Na+, K+) - Group II A elements is +2 (e. g. , Cu 2+, Mg 2+) - Group III A is +3 REDOX continued 13

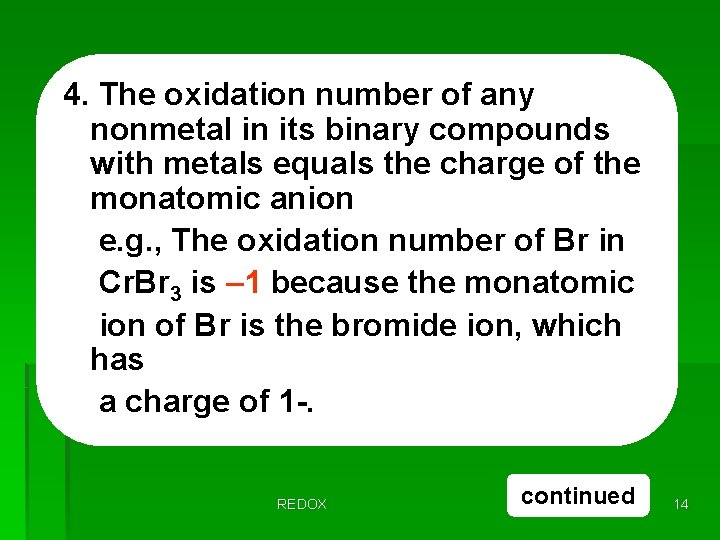
4. The oxidation number of any nonmetal in its binary compounds with metals equals the charge of the monatomic anion e. g. , The oxidation number of Br in Cr. Br 3 is – 1 because the monatomic ion of Br is the bromide ion, which has a charge of 1 -. REDOX continued 14

5. In compounds, the oxidation number of - O is almost always – 2. (Exceptions occur only when the rules for H or F would be violated) - H is almost always +1. (the exceptions are binary compounds with metals, like Na. H, in which the H has the oxidation number – 1. ) - F is always – 1. (No exceptions. Fluorine is the most electronegative of all elements). continued REDOX 15

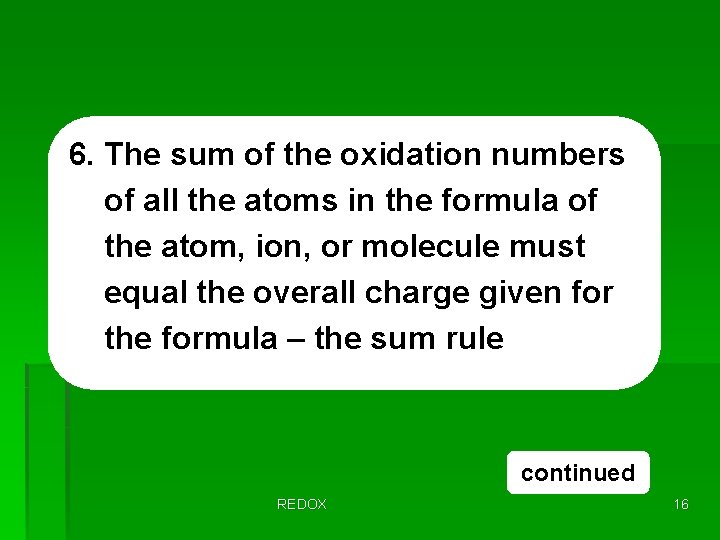
6. The sum of the oxidation numbers of all the atoms in the formula of the atom, ion, or molecule must equal the overall charge given for the formula – the sum rule continued REDOX 16

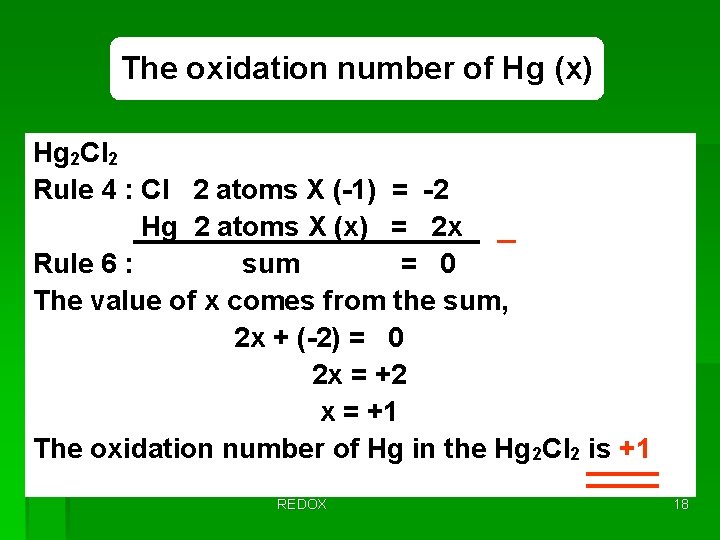
Problem No. 1 Calomel, long use in medicine, has the formula Hg 2 Cl 2. What are the oxidation numbers on the atoms in this compound? The rule for assigning oxidation number: No. 4 & 6 4. The oxidation number of any nonmetal in its binary compounds with metals equals the charge of the monatomic anion 6. The sum of the oxidation numbers of all the atoms in the formula of the atom, ion, or molecule must equal the overall charge given for the formula – the sum rule REDOX 17

The oxidation number of Hg (x) Hg 2 Cl 2 Rule 4 : Cl 2 atoms X (-1) = -2 Hg 2 atoms X (x) = 2 x _ Rule 6 : sum = 0 The value of x comes from the sum, 2 x + (-2) = 0 2 x = +2 x = +1 The oxidation number of Hg in the Hg 2 Cl 2 is +1 REDOX 18

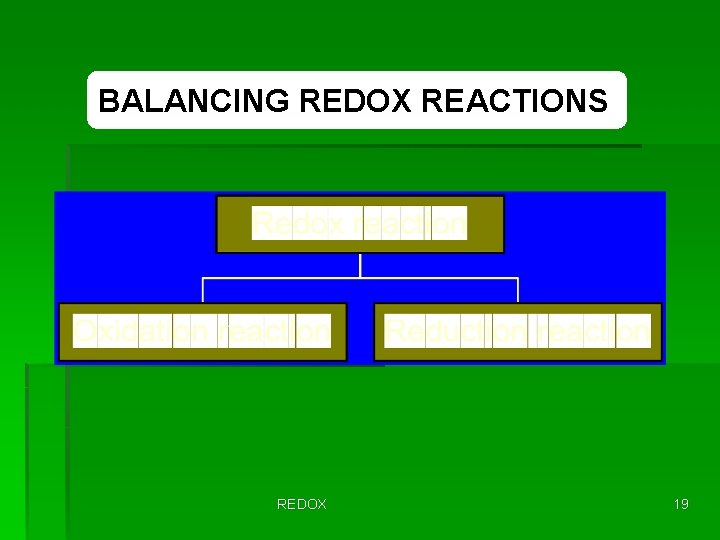
BALANCING REDOX REACTIONS REDOX 19

BALANCING REDOX REACTIONS BY THE ION-ELECTRON METHOD Simply list the steps to balance a redox reaction in an acidic (or neutral) medium. Balancing redox reactions by the ion-electron method has 8 steps. REDOX 20

Those are: 1. Write a skeletal equation that shows only the ions or molecules involved in the reaction. 2. Divide the skeletal equation into two halfreactions. 3. Balance all atoms that are not H or O. 4. Balance O by adding H 2 O. 5. Balance H by adding H+ (not H or H 2 or H¯, but H+). 6. Balance the net charge by adding e¯. (remember its minus sign. ) REDOX continued 21

7. Multiply Entire Half-reactions by simple whole numbers, as needed, to get the gain of e¯ in one halfreaction to match the loss of e¯ in the other. Then add the half-reactions. 8. Cancel whatever is the same on both sides of the arrow. REDOX 22

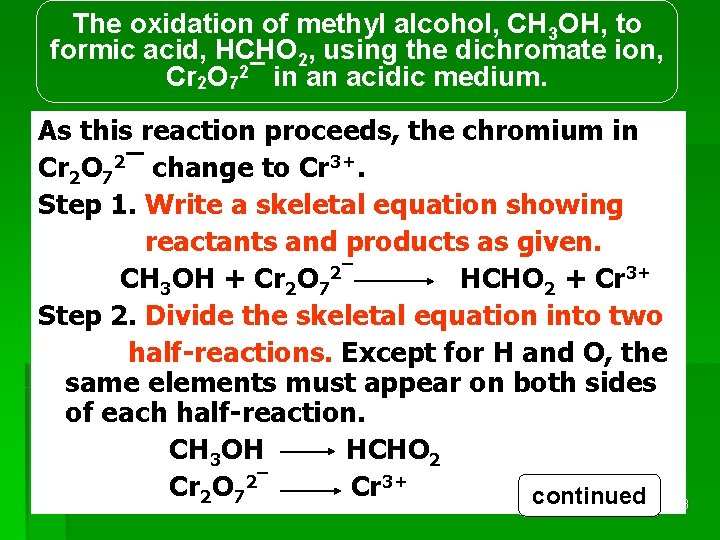
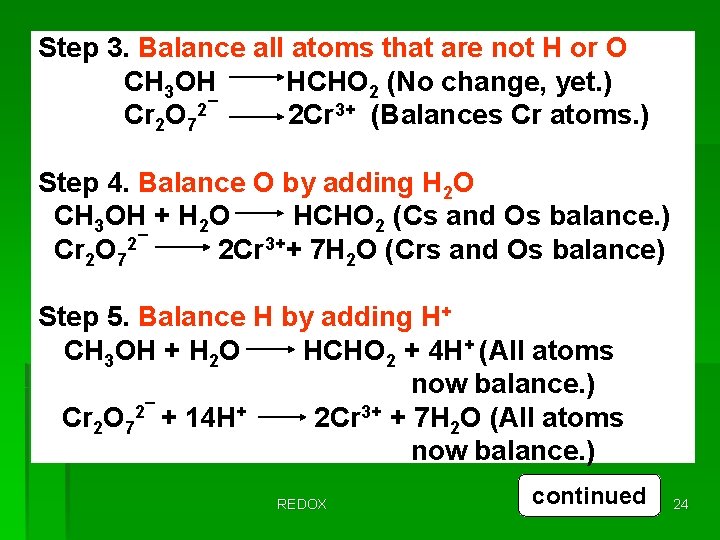
The oxidation of methyl alcohol, CH 3 OH, to formic acid, HCHO 2, using the dichromate ion, Cr 2 O 72¯ in an acidic medium. As this reaction proceeds, the chromium in Cr 2 O 72¯ change to Cr 3+. Step 1. Write a skeletal equation showing reactants and products as given. CH 3 OH + Cr 2 O 72¯ HCHO 2 + Cr 3+ Step 2. Divide the skeletal equation into two half-reactions. Except for H and O, the same elements must appear on both sides of each half-reaction. CH 3 OH HCHO 2 Cr 2 O 72¯ REDOX Cr 3+ continued 23

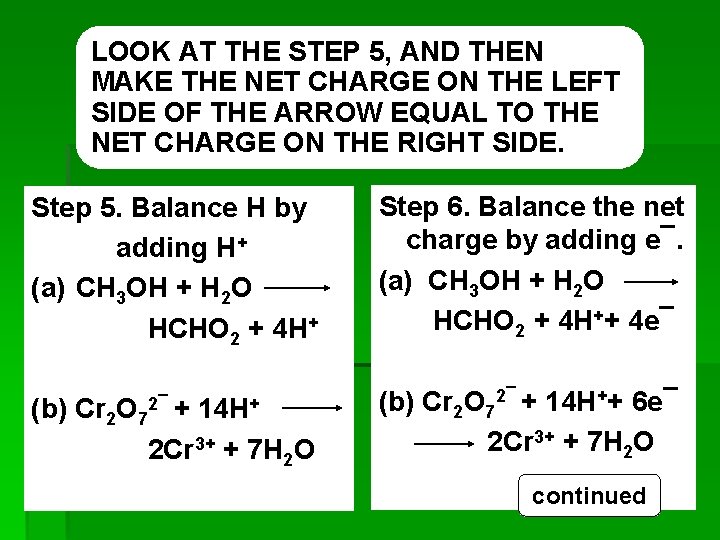
Step 3. Balance all atoms that are not H or O CH 3 OH HCHO 2 (No change, yet. ) Cr 2 O 72¯ 2 Cr 3+ (Balances Cr atoms. ) Step 4. Balance O by adding H 2 O CH 3 OH + H 2 O HCHO 2 (Cs and Os balance. ) Cr 2 O 72¯ 2 Cr 3++ 7 H 2 O (Crs and Os balance) Step 5. Balance H by adding H+ CH 3 OH + H 2 O HCHO 2 + 4 H+ (All atoms now balance. ) Cr 2 O 72¯ + 14 H+ 2 Cr 3+ + 7 H 2 O (All atoms now balance. ) REDOX continued 24

LOOK AT THE STEP 5, AND THEN MAKE THE NET CHARGE ON THE LEFT SIDE OF THE ARROW EQUAL TO THE NET CHARGE ON THE RIGHT SIDE. Step 5. Balance H by adding H+ (a) CH 3 OH + H 2 O HCHO 2 + 4 H+ 2¯ + 14 H+ (b) Cr 2 O 7 2 Cr 3+ + 7 H 2 O REDOX Step 6. Balance the net charge by adding e¯. (a) CH 3 OH + H 2 O HCHO 2 + 4 H++ 4 e¯ (b) Cr 2 O 72¯ + 14 H++ 6 e¯ 2 Cr 3+ + 7 H 2 O continued 25

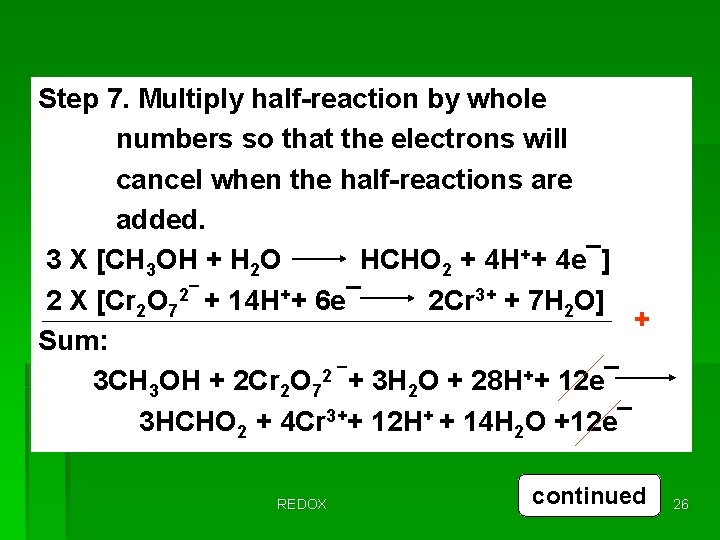
Step 7. Multiply half-reaction by whole numbers so that the electrons will cancel when the half-reactions are added. 3 X [CH 3 OH + H 2 O HCHO 2 + 4 H++ 4 e¯] 2 X [Cr 2 O 72¯ + 14 H++ 6 e¯ 2 Cr 3+ + 7 H 2 O] + Sum: 3 CH 3 OH + 2 Cr 2 O 72 ¯+ 3 H 2 O + 28 H++ 12 e¯ 3 HCHO 2 + 4 Cr 3++ 12 H+ + 14 H 2 O +12 e¯ REDOX continued 26

Step 8. Cancel everything that can be canceled a). The 12 electrons on each side obviously cancel. b). Water molecule: there are 3 on the left and 14 on the right, so we can strike those on the left and change those on the right to 11. …. + 3 H 2 O +…. …. + 14 H 2 O +…. becomes: …. . … + 11 H 2 O + …. c). And then also cancel some H+ …. + 28 H+ + …. …. + 12 H+ + …. becomes: …. + 16 H+ + …. …. . REDOX continued 27

3 CH 3 OH(aq) + 2 Cr 2 O 72 ¯(aq) + 16 H+(aq) 3 HCHO 2(aq) + 4 Cr 3+(aq) +11 H 2 O Check to see that both material and electrical balance exist. REDOX 28

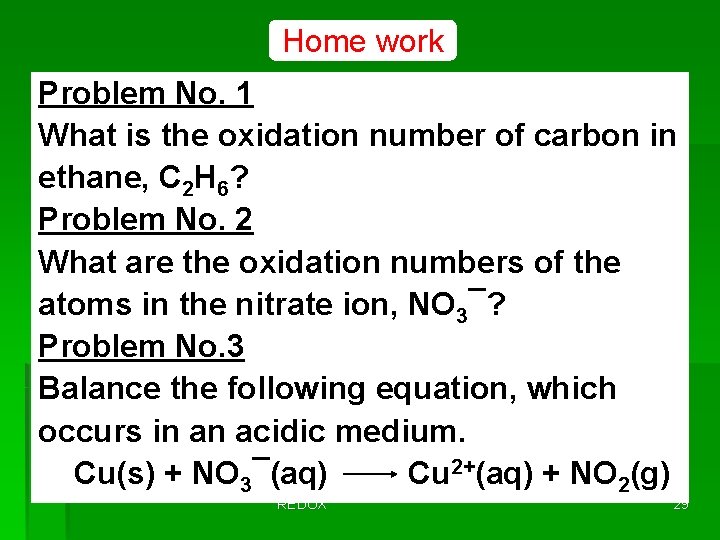
Home work Problem No. 1 What is the oxidation number of carbon in ethane, C 2 H 6? Problem No. 2 What are the oxidation numbers of the atoms in the nitrate ion, NO 3¯? Problem No. 3 Balance the following equation, which occurs in an acidic medium. Cu(s) + NO 3¯(aq) Cu 2+(aq) + NO 2(g) REDOX 29

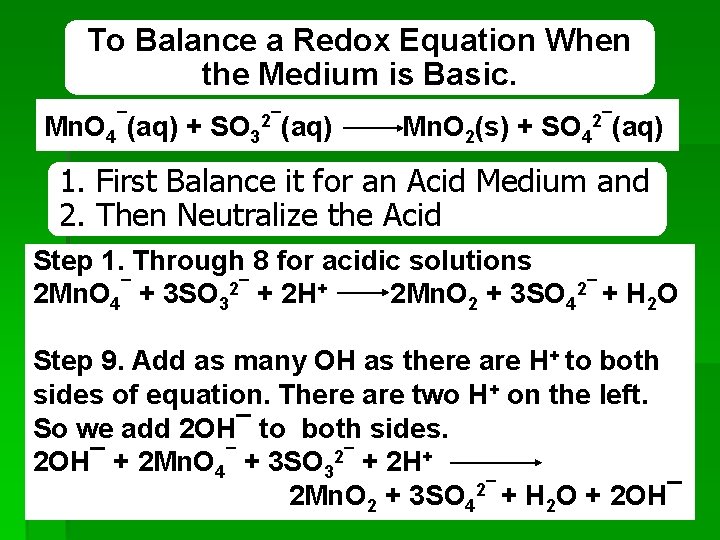
To Balance a Redox Equation When the Medium is Basic. Mn. O 4¯(aq) + SO 32¯(aq) Mn. O 2(s) + SO 42¯(aq) 1. First Balance it for an Acid Medium and 2. Then Neutralize the Acid Step 1. Through 8 for acidic solutions 2 Mn. O 4¯ + 3 SO 32¯ + 2 H+ 2 Mn. O 2 + 3 SO 42¯ + H 2 O Step 9. Add as many OH as there are H+ to both sides of equation. There are two H+ on the left. So we add 2 OH¯ to both sides. 2 OH¯ + 2 Mn. O 4¯ + 3 SO 32¯ + 2 H+ 2 Mn. O 2 + 3 SO 42¯ + H 2 O + 2 OH¯ 30 REDOX

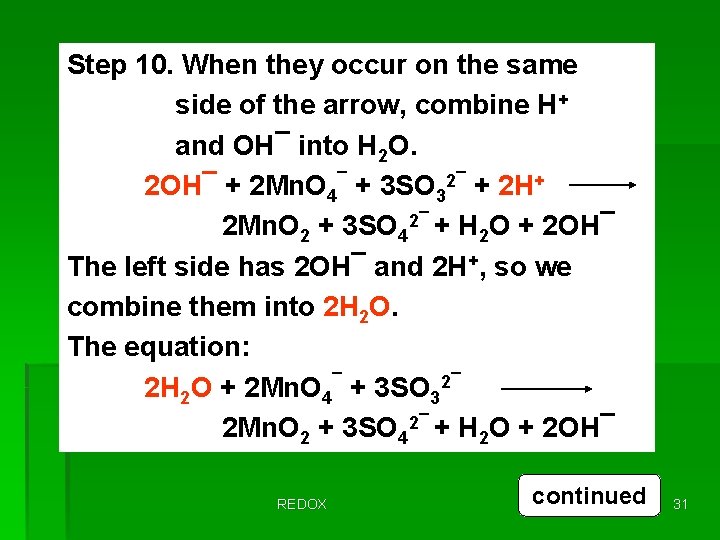
Step 10. When they occur on the same side of the arrow, combine H+ and OH¯ into H 2 O. 2 OH¯ + 2 Mn. O 4¯ + 3 SO 32¯ + 2 H+ 2 Mn. O 2 + 3 SO 42¯ + H 2 O + 2 OH¯ The left side has 2 OH¯ and 2 H+, so we combine them into 2 H 2 O. The equation: 2 H 2 O + 2 Mn. O 4¯ + 3 SO 32¯ 2 Mn. O 2 + 3 SO 42¯ + H 2 O + 2 OH¯ REDOX continued 31

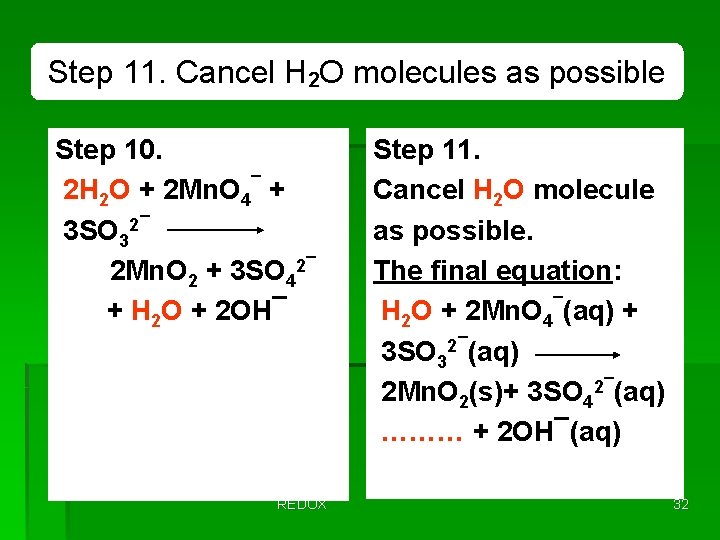
Step 11. Cancel H 2 O molecules as possible Step 10. 2 H 2 O + 2 Mn. O 4¯ + 3 SO 32¯ 2 Mn. O 2 + 3 SO 42¯ + H 2 O + 2 OH¯ REDOX Step 11. Cancel H 2 O molecule as possible. The final equation: H 2 O + 2 Mn. O 4¯(aq) + 3 SO 32¯(aq) 2 Mn. O 2(s)+ 3 SO 42¯(aq) ……… + 2 OH¯(aq) 32

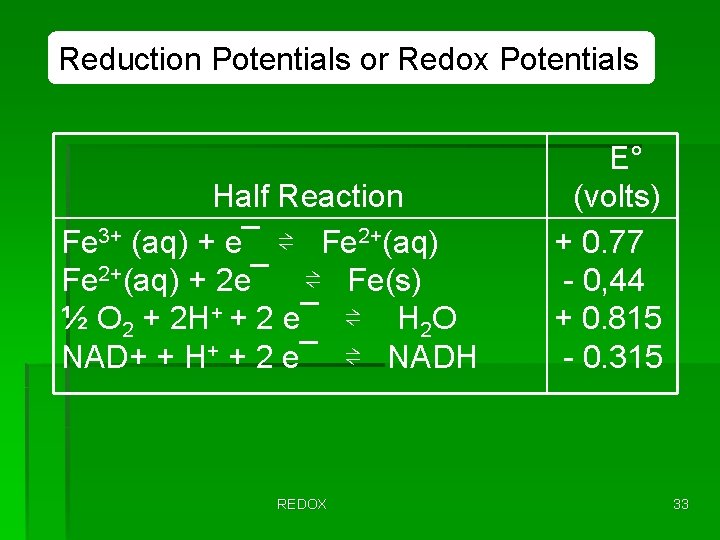
Reduction Potentials or Redox Potentials Half Reaction Fe 3+ (aq) + e¯ ⇌ Fe 2+(aq) + 2 e¯ ⇌ Fe(s) ½ O 2 + 2 H+ + 2 e¯ ⇌ H 2 O NAD+ + H+ + 2 e¯ ⇌ NADH REDOX E° (volts) + 0. 77 - 0, 44 + 0. 815 - 0. 315 33

Rule for Combining Reduction Half-Reactions When two reduction halfreactions are combined into a full redox reaction the one with the more positive E° always runs as written, as a reduction, and it forces the other, with the less positive E°, to run in reverse, as an oxidation. REDOX 34

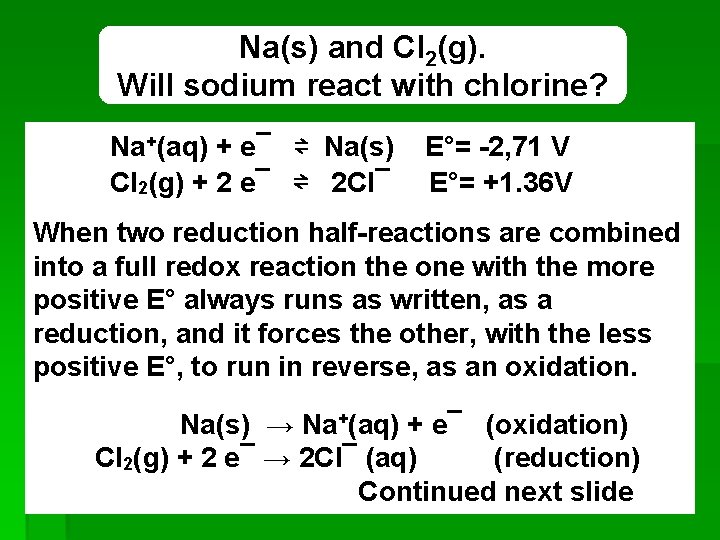
Na(s) and Cl 2(g). Will sodium react with chlorine? Na+(aq) + e¯ ⇌ Na(s) Cl 2(g) + 2 e¯ ⇌ 2 Cl¯ E°= -2, 71 V E°= +1. 36 V When two reduction half-reactions are combined into a full redox reaction the one with the more positive E° always runs as written, as a reduction, and it forces the other, with the less positive E°, to run in reverse, as an oxidation. Na(s) → Na+(aq) + e¯ (oxidation) Cl 2(g) + 2 e¯ → 2 Cl¯ (aq) (reduction) Continued next slide REDOX 35

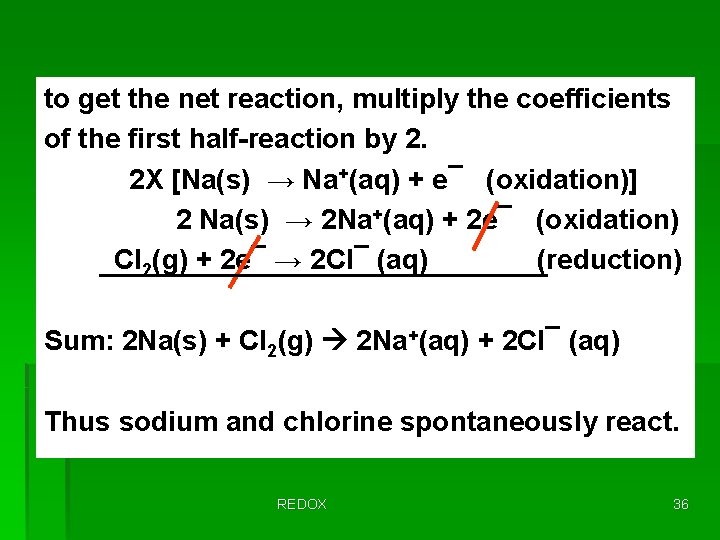
to get the net reaction, multiply the coefficients of the first half-reaction by 2. 2 X [Na(s) → Na+(aq) + e¯ (oxidation)] 2 Na(s) → 2 Na+(aq) + 2 e¯ (oxidation) Cl 2(g) + 2 e¯ → 2 Cl¯ (aq) (reduction) Sum: 2 Na(s) + Cl 2(g) 2 Na+(aq) + 2 Cl¯ (aq) Thus sodium and chlorine spontaneously react. REDOX 36

TYPE OF REDOX REACTION § OUT OF BODY REDOX § IN THE BODY 37

Biological Oxidation § The processes of oxidation are essential for maintaining life because oxidation and the simultaneously occuring reduction supply the free energy for the vital work REDOX 38

Organic Reaction Mechanisms § Organic reactions mechanisms has classified biochemical reactions into four categories: § 1. Group-transfer reactions § 2. Oxidations and reductions § 3. Eliminations, isomerizations, and rearrangements § 4. Reactions that make or break carbon bonds REDOX 39

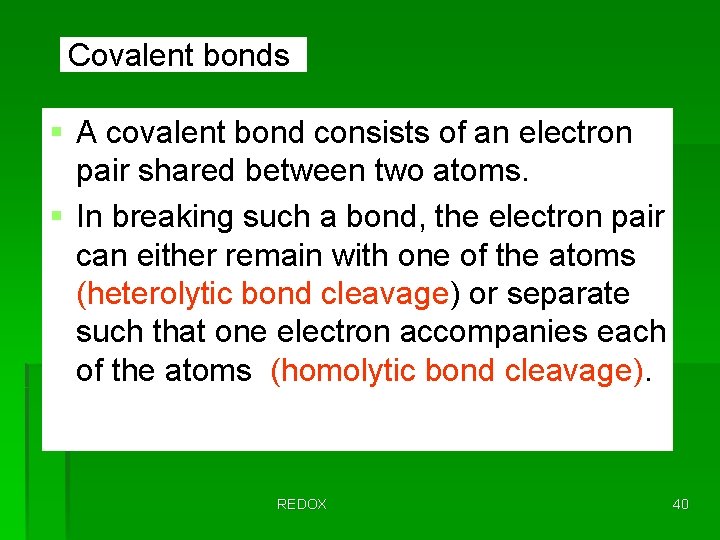
Covalent bonds § A covalent bond consists of an electron pair shared between two atoms. § In breaking such a bond, the electron pair can either remain with one of the atoms (heterolytic bond cleavage) or separate such that one electron accompanies each of the atoms (homolytic bond cleavage). REDOX 40

Homolytic Bond Cleavage § Homolytic bond cleavage, which usually produces unstable radicals, occurs mostly in oxidation-reduction reactions REDOX 41

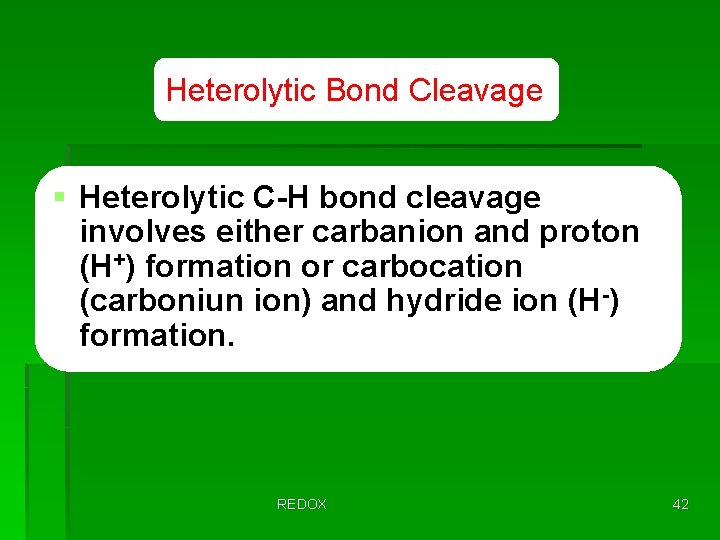
Heterolytic Bond Cleavage § Heterolytic C-H bond cleavage involves either carbanion and proton (H+) formation or carbocation (carboniun ion) and hydride ion (H-) formation. REDOX 42

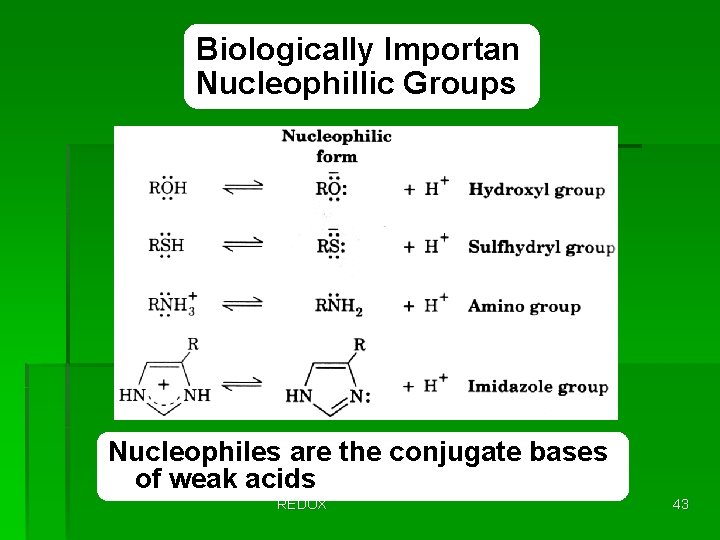
Biologically Importan Nucleophillic Groups Nucleophiles are the conjugate bases of weak acids REDOX 43

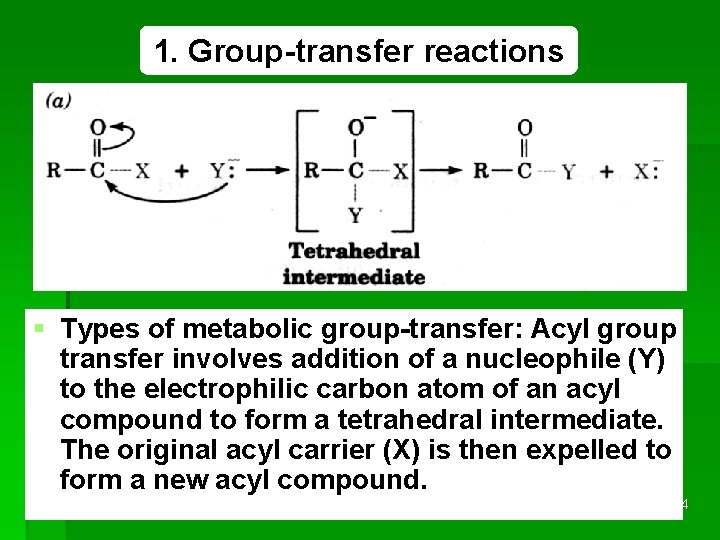
1. Group-transfer reactions § Types of metabolic group-transfer: Acyl group transfer involves addition of a nucleophile (Y) to the electrophilic carbon atom of an acyl compound to form a tetrahedral intermediate. The original acyl carrier (X) is then expelled to form a new acyl compound. REDOX 44

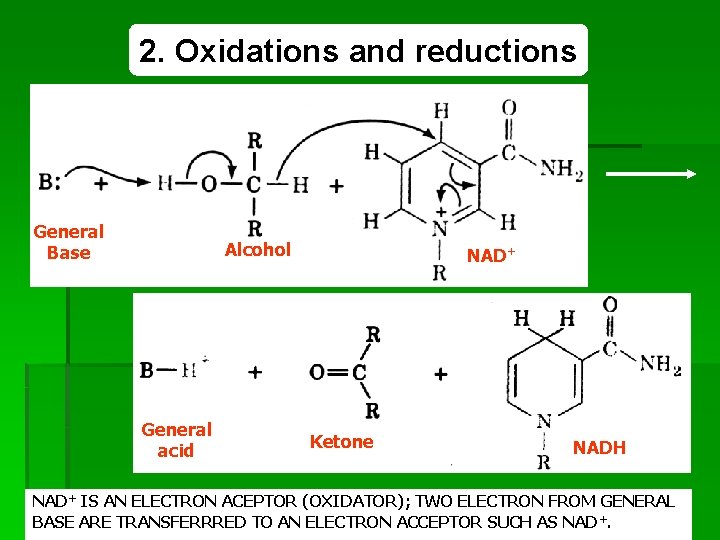
2. Oxidations and reductions General Base Alcohol General acid NAD+ Ketone NADH NAD+ IS AN ELECTRON ACEPTOR (OXIDATOR); TWO ELECTRON FROM GENERAL 45 REDOX BASE ARE TRANSFERRRED TO AN ELECTRON ACCEPTOR SUCH AS NAD+.

In Living Systems The electron-transfer process connecting these half-reactions occurs through a multistep pathway that harnesses the liberated free energy to form ATP. REDOX 46

For Example: Biologic Oxidation § The principal use of oxygen is in respiration. Which may be defined as the process by with cells derive energy in the form of ATP from the controlled reaction of hydrogen with oxygen to form water. REDOX 47

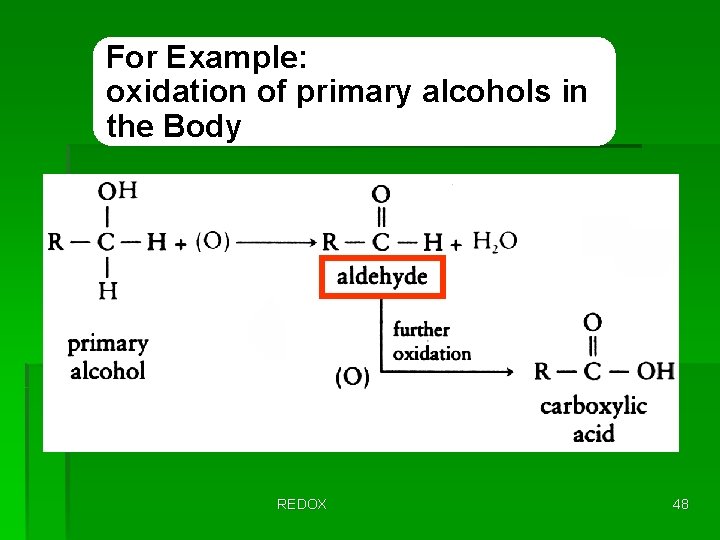
For Example: oxidation of primary alcohols in the Body REDOX 48

ACCUT TOXICITY Toxicity test SUBCHRONIC TOXICITY REDOX 49

FREE ENERGY CHANGES CAN BE EXPRESSED IN TERMS OF REDOX POTENTIAL § In reaction involving oxidation and reduction, the free energy change is proportionate to the tendency of reactants to donate or accept electrons. Thus, in addition to expressing free energy change in terms of ΔG 0. § It is possible, in an analogous manner, to express it numerically as an oxidationreduction or redox potential (E 0). REDOX 50

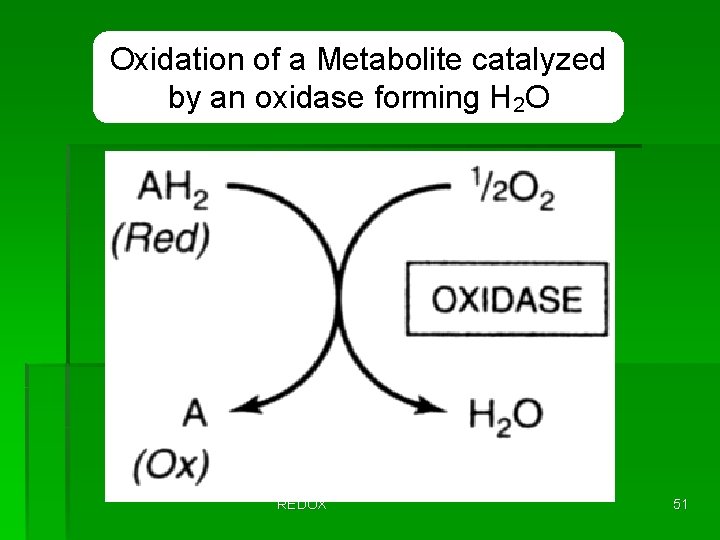
Oxidation of a Metabolite catalyzed by an oxidase forming H 2 O REDOX 51

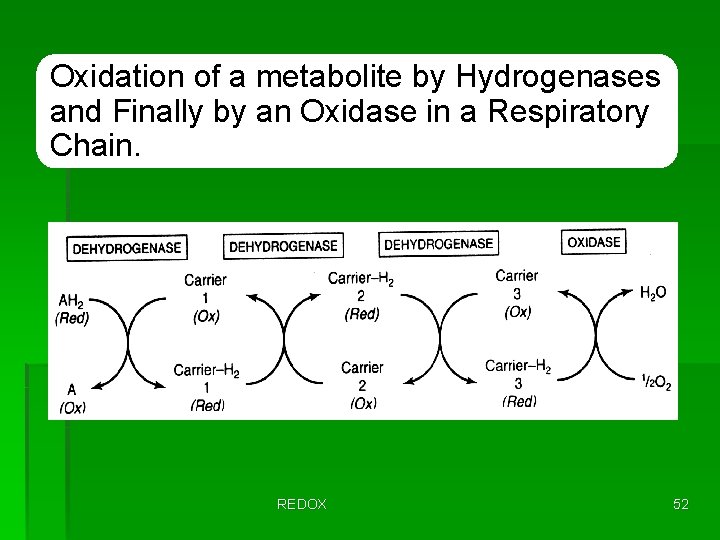
Oxidation of a metabolite by Hydrogenases and Finally by an Oxidase in a Respiratory Chain. REDOX 52

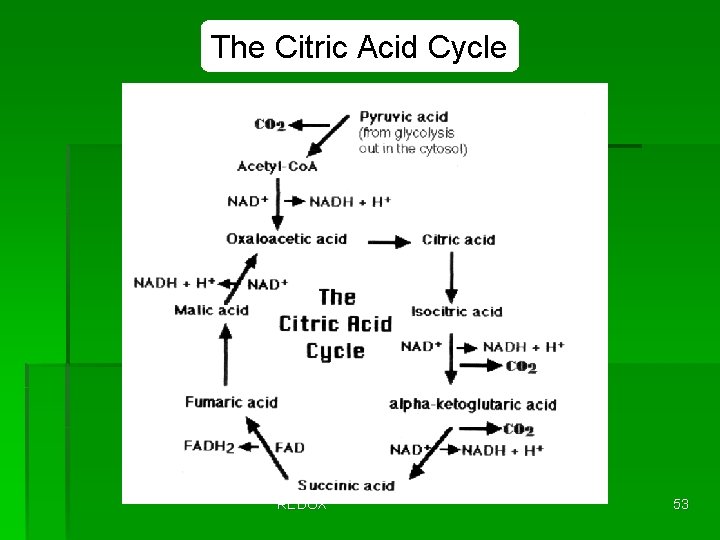
The Citric Acid Cycle REDOX 53

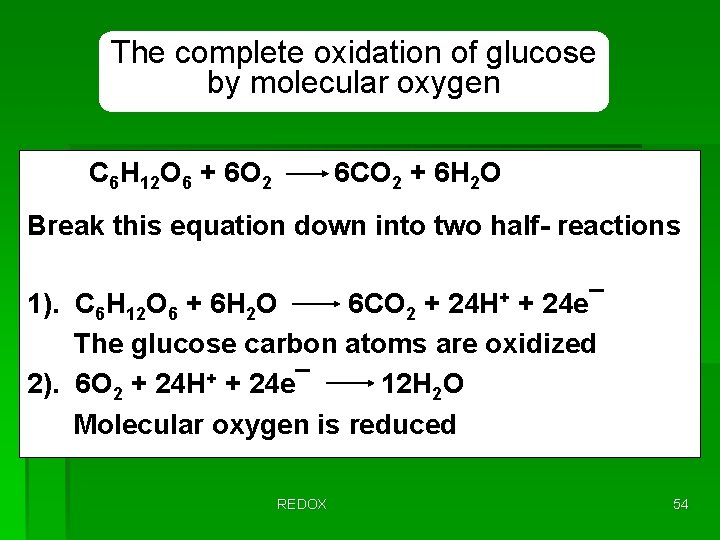
The complete oxidation of glucose by molecular oxygen C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O Break this equation down into two half- reactions 1). C 6 H 12 O 6 + 6 H 2 O 6 CO 2 + 24 H+ + 24 e¯ The glucose carbon atoms are oxidized 2). 6 O 2 + 24 H+ + 24 e¯ 12 H 2 O Molecular oxygen is reduced REDOX 54

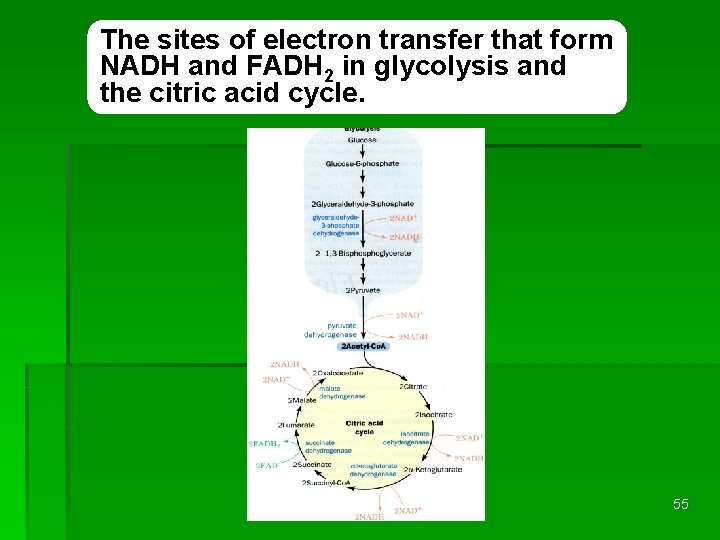
The sites of electron transfer that form NADH and FADH 2 in glycolysis and the citric acid cycle. REDOX 55

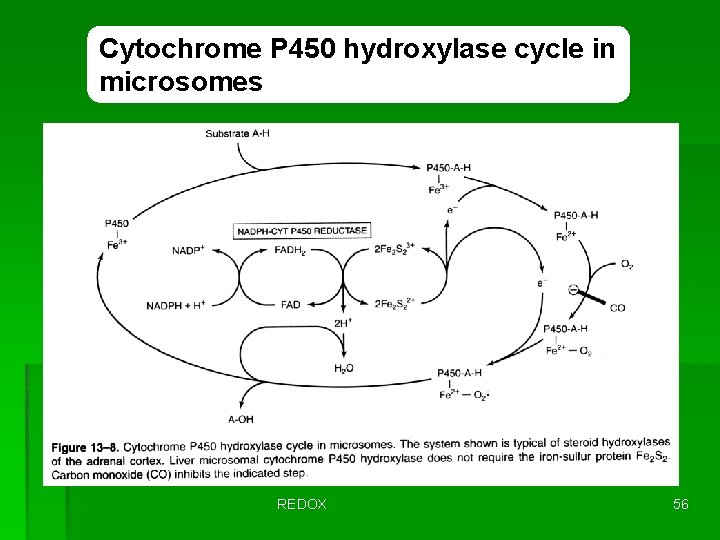
Cytochrome P 450 hydroxylase cycle in microsomes REDOX 56

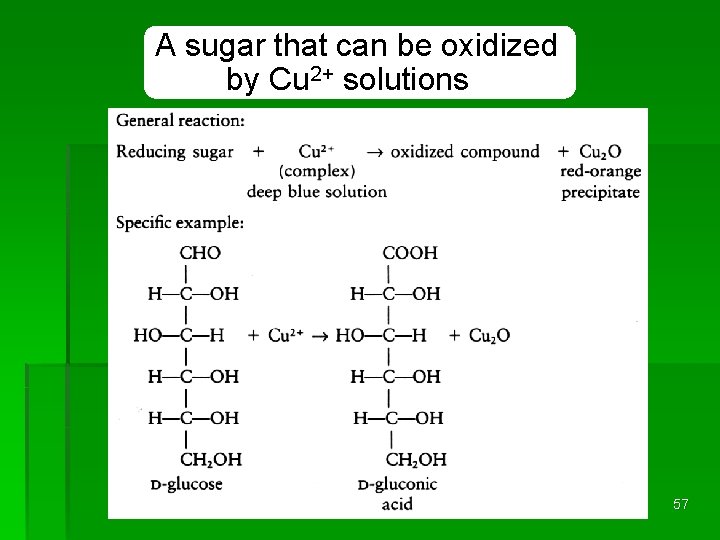
A sugar that can be oxidized by Cu 2+ solutions REDOX 57