OXIDATION AND REDUCTION Dr Vs AP Chemistry Webcasts

OXIDATION AND REDUCTION Dr V’s AP Chemistry Webcasts

OBJECTIVES • What happens during oxidation? • What happens during reduction? • Oxidation and reduction half-reactions • Compare redox vs. non-redox reactions

OXIDATION • Oxidation = loss of electrons • In oxidation, the oxidation number increases • Ex: Na loses one electron to form Na+ Na Na+ e-
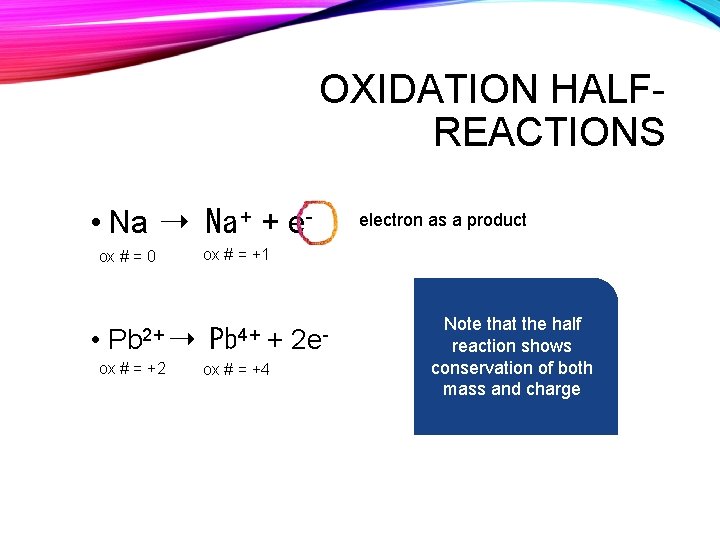
OXIDATION HALFREACTIONS • Na ➝ Na + + eox # = 0 • Pb 2+ ➝ ox # = +2 electron as a product ox # = +1 Pb 4+ + ox # = +4 2 e- Note that the half reaction shows conservation of both mass and charge
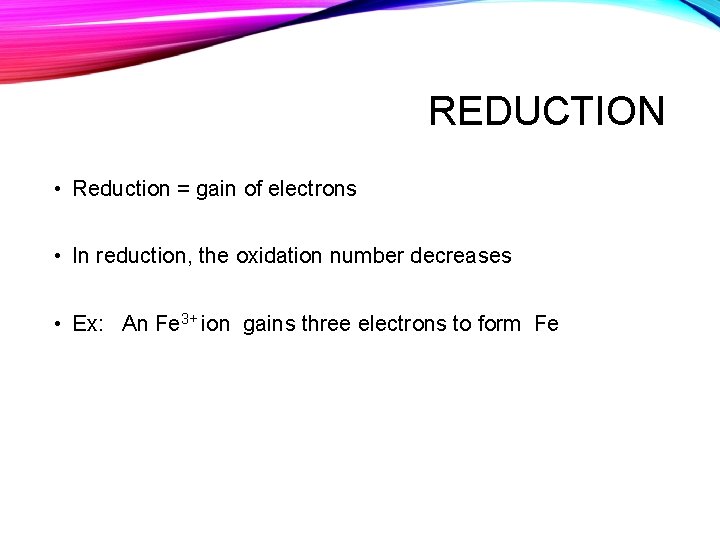
REDUCTION • Reduction = gain of electrons • In reduction, the oxidation number decreases • Ex: An Fe 3+ ion gains three electrons to form Fe e- e 3+ Fe. Fe e-
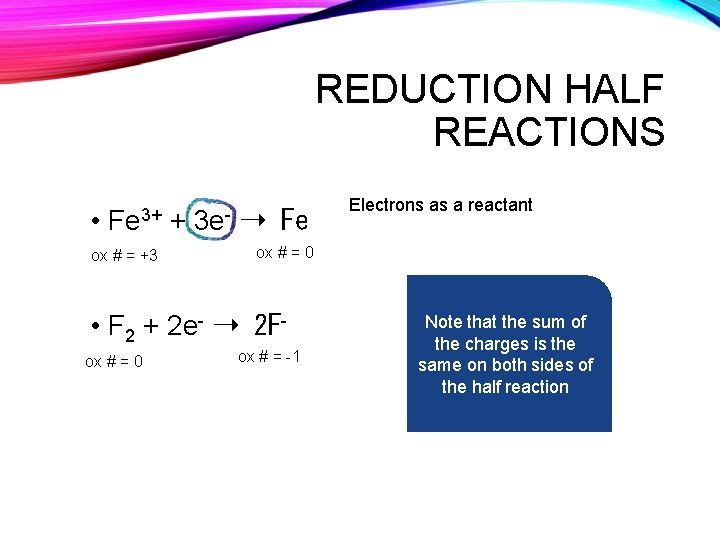
REDUCTION HALF REACTIONS • Fe 3+ ox # = +3 + 3 e- ➝ Fe ox # = 0 • F 2 + 2 e- ➝ 2 Fox # = 0 Electrons as a reactant ox # = -1 Note that the sum of the charges is the same on both sides of the half reaction

MNEMONICS • LEO the lion says GER • OIL RIG

REDOX REACTIONS • Oxidation can’t occur without reduction! • The number of electrons lost must equal the number of electrons gained. • Conservation of charge • In a redox reaction, oxidation numbers change.

ARE THESE REDOX REACTIONS? +2 -2 0 0 +1 -2 Yes, redox • Cu. O + H 2 ➝ Cu + H 2 O +1 +6 -2 +1 -1 +1 +1 +6 -2 +1 -2 • H 2 SO 4 + 2 Na. OH ➝ Na 2 SO 4 + H 2 O Not redox

ARE THESE REDOX REACTIONS? +2 +4 -2 +2 -2 +4 -2 • Mg. CO 3 ➝ Mg. O + CO +5 -2 +2 -2 0 2 +4 -2 • I 2 O 5 + CO ➝ I 2 + CO 2 0 0 +5 -2 • P 4 + S 8 ➝ P 2 S 5 Not redox Yes, redox
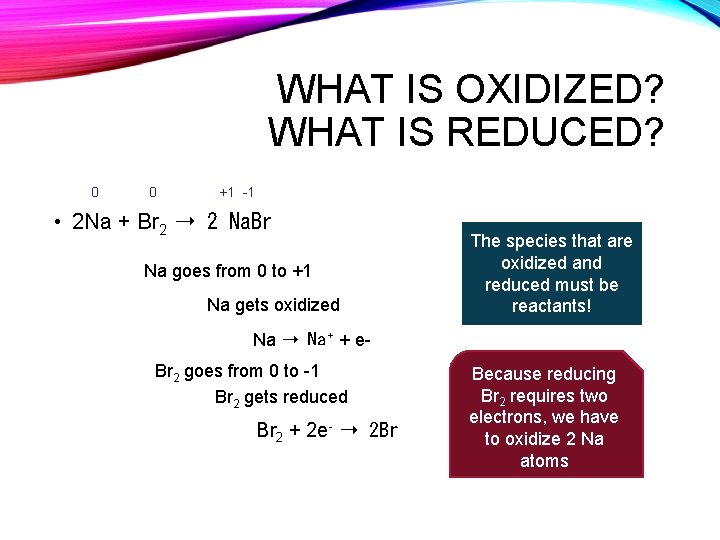
WHAT IS OXIDIZED? WHAT IS REDUCED? 0 0 +1 -1 • 2 Na + Br 2 ➝ 2 Na. Br Na goes from 0 to +1 Na gets oxidized The species that are oxidized and reduced must be reactants! Na ➝ Na + + e. Br 2 goes from 0 to -1 Br 2 gets reduced Br 2 + 2 e- ➝ 2 Br- Because reducing Br 2 requires two electrons, we have to oxidize 2 Na atoms
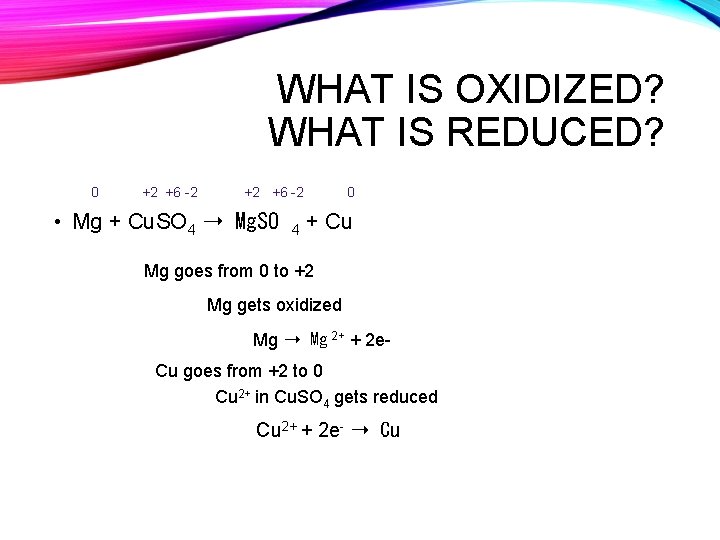
WHAT IS OXIDIZED? WHAT IS REDUCED? 0 +2 +6 -2 • Mg + Cu. SO 4 ➝ Mg. SO 4 0 + Cu Mg goes from 0 to +2 Mg gets oxidized Mg ➝ Mg 2+ + 2 e. Cu goes from +2 to 0 Cu 2+ in Cu. SO 4 gets reduced Cu 2+ + 2 e- ➝ Cu

MORE ABOUT REDOX REACTIONS • Oxidizing agent = the substance that gets reduced • Reducing agent = the substance that gets oxidized Substances that have a strong tendency to undergo reduction (ex. compounds with Mn. O 4 -) are strong oxidizing agents and need to be stored separately!
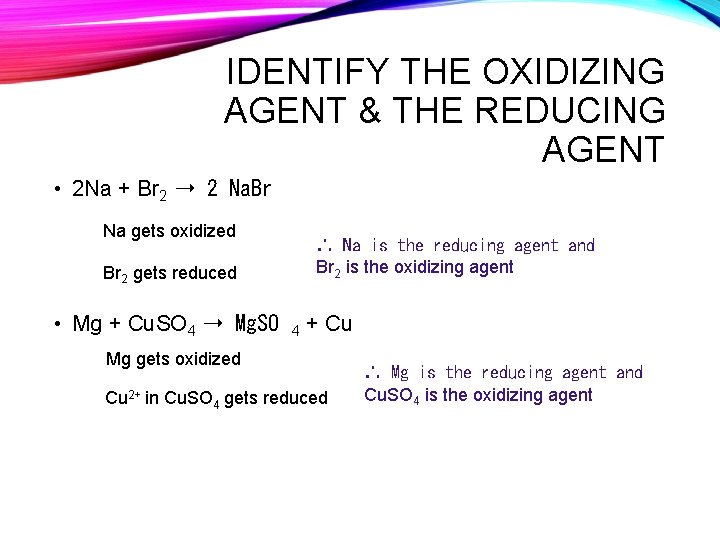
IDENTIFY THE OXIDIZING AGENT & THE REDUCING AGENT • 2 Na + Br 2 ➝ 2 Na. Br Na gets oxidized ∴ Na is the reducing agent and Br 2 is the oxidizing agent Br 2 gets reduced • Mg + Cu. SO 4 ➝ Mg. SO 4 + Cu Mg gets oxidized Cu 2+ in Cu. SO 4 gets reduced ∴ Mg is the reducing agent and Cu. SO 4 is the oxidizing agent
SUMMARY • Oxidation is a loss of electrons. • Reduction is a gain of electrons. • Oxidation numbers change in redox reactions. Subscribe to my channel! Like the video & leave a comment
- Slides: 15