Oxidation and reduction Definitions Oxidation is gain of



- Slides: 7

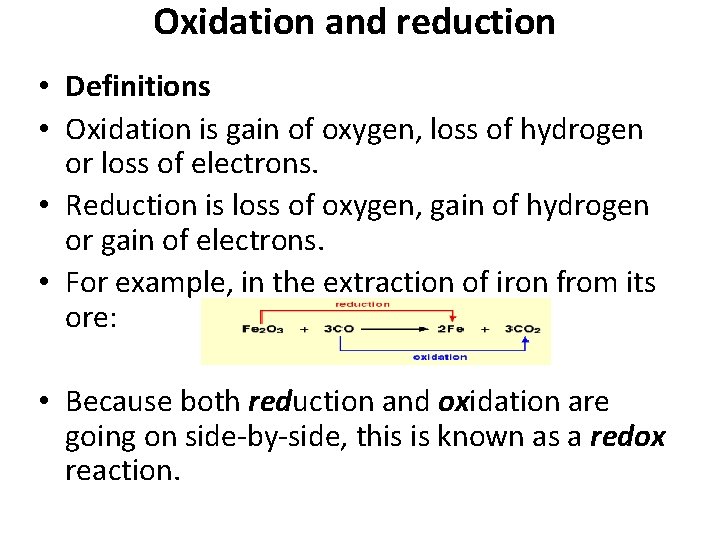
Oxidation and reduction • Definitions • Oxidation is gain of oxygen, loss of hydrogen or loss of electrons. • Reduction is loss of oxygen, gain of hydrogen or gain of electrons. • For example, in the extraction of iron from its ore: • Because both reduction and oxidation are going on side-by-side, this is known as a redox reaction.

REDOX – simultaneous reduction and oxidation REDOX reactions: Oxidation is loss of electrons Reduction is gain of electrons

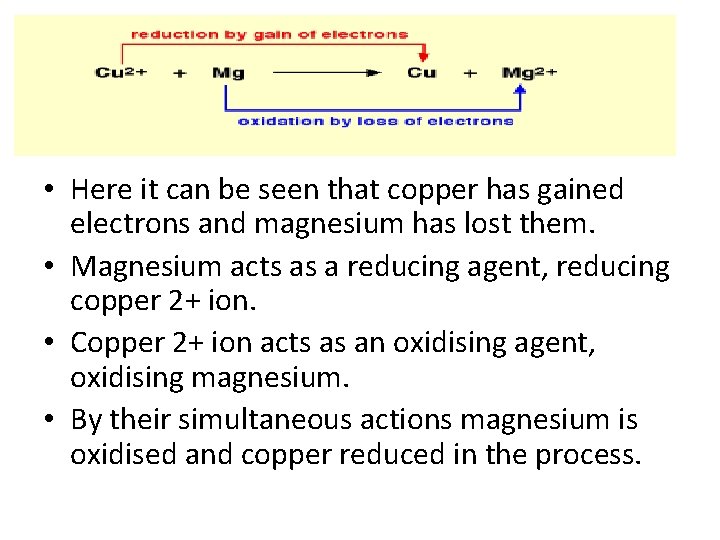
• Here it can be seen that copper has gained electrons and magnesium has lost them. • Magnesium acts as a reducing agent, reducing copper 2+ ion. • Copper 2+ ion acts as an oxidising agent, oxidising magnesium. • By their simultaneous actions magnesium is oxidised and copper reduced in the process.

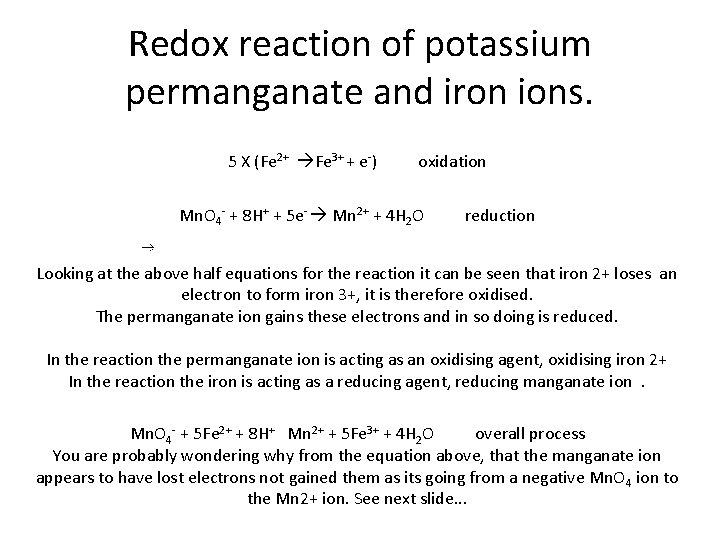
Redox reaction of potassium permanganate and iron ions. 5 X (Fe 2+ Fe 3+ + e-) oxidation Mn. O 4 - + 8 H+ + 5 e- Mn 2+ + 4 H 2 O reduction Looking at the above half equations for the reaction it can be seen that iron 2+ loses an electron to form iron 3+, it is therefore oxidised. The permanganate ion gains these electrons and in so doing is reduced. In the reaction the permanganate ion is acting as an oxidising agent, oxidising iron 2+ In the reaction the iron is acting as a reducing agent, reducing manganate ion . Mn. O 4 - + 5 Fe 2+ + 8 H+ Mn 2+ + 5 Fe 3+ + 4 H 2 O overall process You are probably wondering why from the equation above, that the manganate ion appears to have lost electrons not gained them as its going from a negative Mn. O 4 ion to the Mn 2+ ion. See next slide. . .

Iron’s changing oxidation state • Iron changes from +2 to +3 • (Fe 2+ Fe 3+ + e-) oxidation • This means its oxidation state (the charge on the ion) changes from +2 to +3, this is the loss of an electron: • The nucleus on the iron 2+ ion has a surplus of 2 protons to the number of electrons in the atom. • In the reaction this changes as iron loses a further electron, so now forms a +3 ion. • The number of protons now outweighs the number of electrons by 3 instead of 2 to account for this loss of a further electron. • Iron is oxidised (oxidation is loss of electrons)

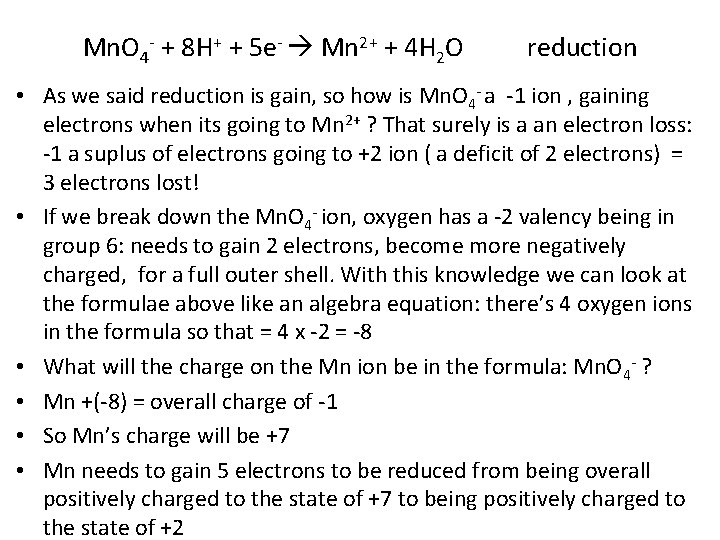
Mn. O 4 - + 8 H+ + 5 e- Mn 2+ + 4 H 2 O reduction • As we said reduction is gain, so how is Mn. O 4 - a -1 ion , gaining electrons when its going to Mn 2+ ? That surely is a an electron loss: -1 a suplus of electrons going to +2 ion ( a deficit of 2 electrons) = 3 electrons lost! • If we break down the Mn. O 4 - ion, oxygen has a -2 valency being in group 6: needs to gain 2 electrons, become more negatively charged, for a full outer shell. With this knowledge we can look at the formulae above like an algebra equation: there’s 4 oxygen ions in the formula so that = 4 x -2 = -8 • What will the charge on the Mn ion be in the formula: Mn. O 4 - ? • Mn +(-8) = overall charge of -1 • So Mn’s charge will be +7 • Mn needs to gain 5 electrons to be reduced from being overall positively charged to the state of +7 to being positively charged to the state of +2

Oxidation states • The loss or gain of electrons that causes a change in the charge of an ion as a result of a redox reaction causes a change in the oxidation state or charge on the ion. • Most transition metals will have more than one oxidation state: e. g. Mn = +7 and +2 oxidation states, iron = +2 or +3 oxidation states also 0 when its in its metallic form, as electrons and protons are balanced. Copper is a further example existing in 0 as the metal and +1 and +2 as ions. Thinking back to the Feiling’s reaction with aldehydes, we see copper reduced from the blue solution of Cu 2+ ions, +2 state to the red precipitate of Cu +, the +1 state. • The reason we see different colours in transition metal ions is due to them having d orbitals where electrons can move about and emit photons of light with different amounts of energy, that is emitted in the visible region of the electromagnetic spectrum, this changes depending on the number of electrons in d orbitals / or changes in oxidation state. For more information on this topic click the link below: ( for curiosity only, not necessary knowledge for the assignment!) http: //www. chemguide. co. uk/inorganic/complexions/colour. html