Oxidants in organic synthesis Initiators of freeradical reactions






- Slides: 28


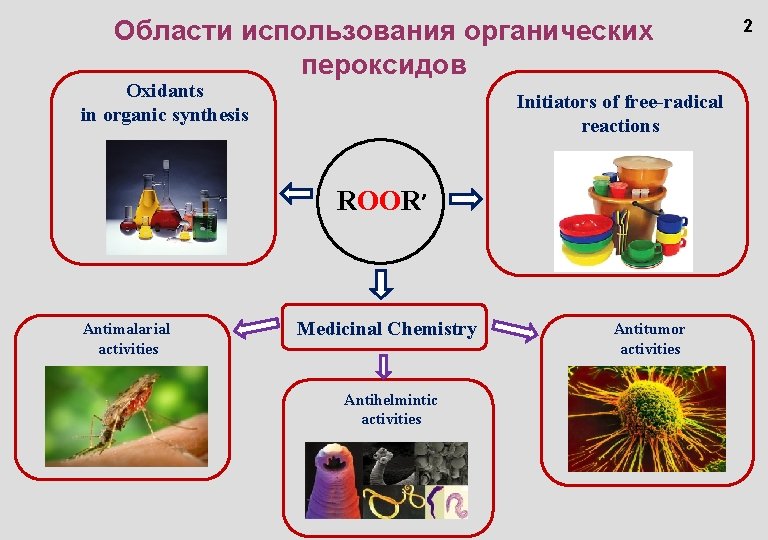
Области использования органических пероксидов Oxidants in organic synthesis Initiators of free-radical reactions ROOR’ Antimalarial activities Medicinal Chemistry Antihelmintic activities Antitumor activities 2

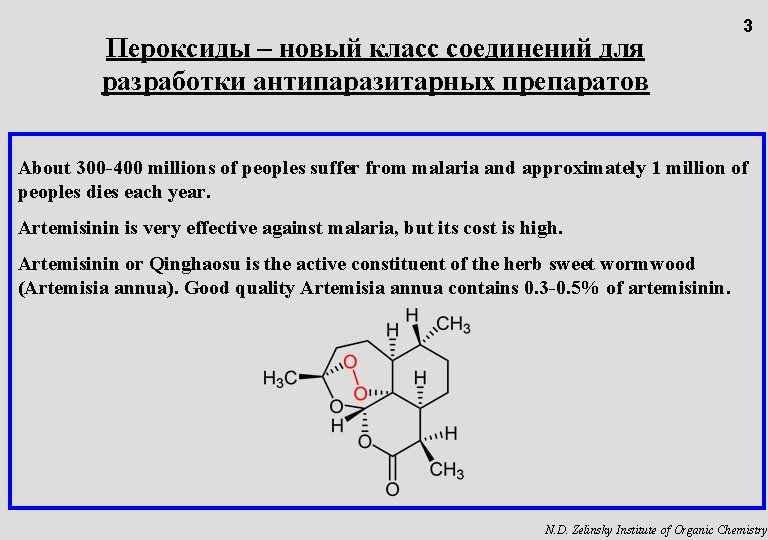
Пероксиды – новый класс соединений для разработки антипаразитарных препаратов 3 About 300 -400 millions of peoples suffer from malaria and approximately 1 million of peoples dies each year. Artemisinin is very effective against malaria, but its cost is high. Artemisinin or Qinghaosu is the active constituent of the herb sweet wormwood (Artemisia annua). Good quality Artemisia annua contains 0. 3 -0. 5% of artemisinin. N. D. Zelinsky Institute of Organic Chemistry

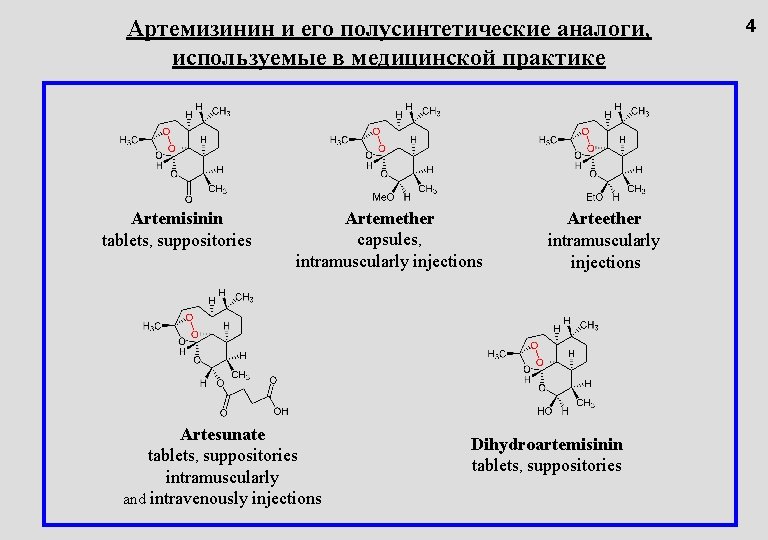
Артемизинин и его полусинтетические аналоги, используемые в медицинской практике Artemisinin tablets, suppositories Artemether capsules, intramuscularly injections Artesunate tablets, suppositories intramuscularly and intravenously injections Arteether intramuscularly injections Dihydroartemisinin tablets, suppositories 4

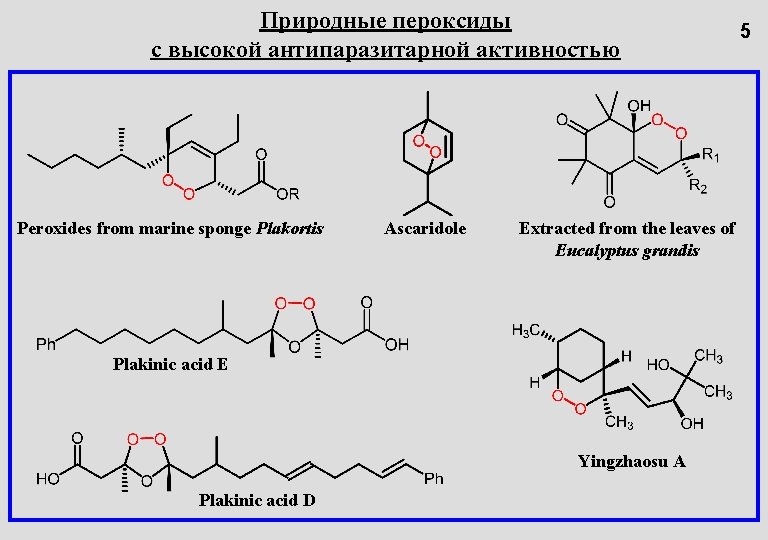
Природные пероксиды с высокой антипаразитарной активностью Peroxides from marine sponge Plakortis Ascaridole Extracted from the leaves of Eucalyptus grandis Plakinic acid E Yingzhaosu A Plakinic acid D 5

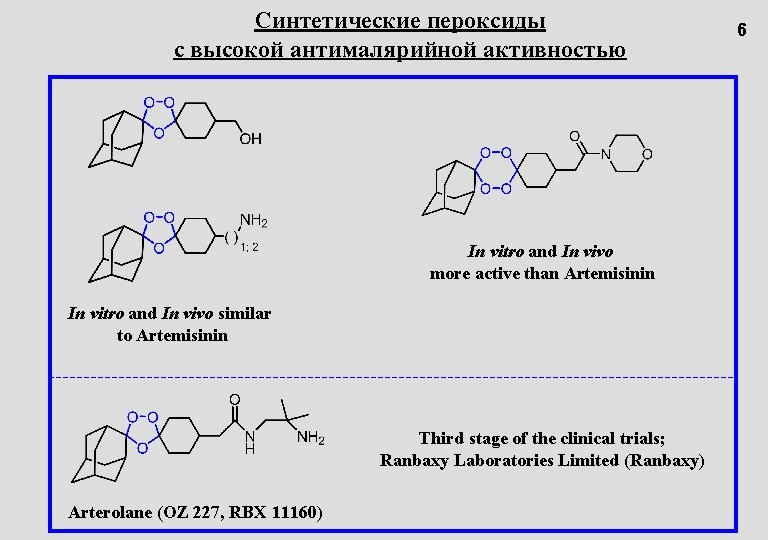
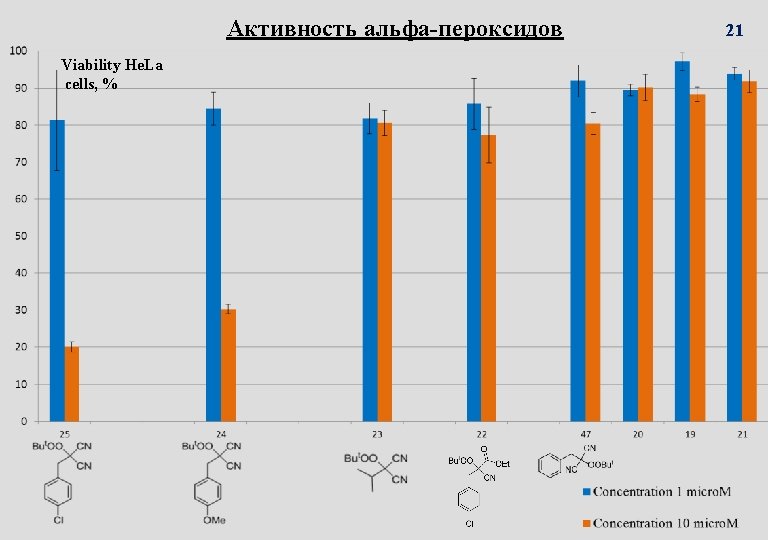
Синтетические пероксиды с высокой антималярийной активностью In vitro and In vivo more active than Artemisinin In vitro and In vivo similar to Artemisinin Third stage of the clinical trials; Ranbaxy Laboratories Limited (Ranbaxy) Arterolane (OZ 227, RBX 11160) 6

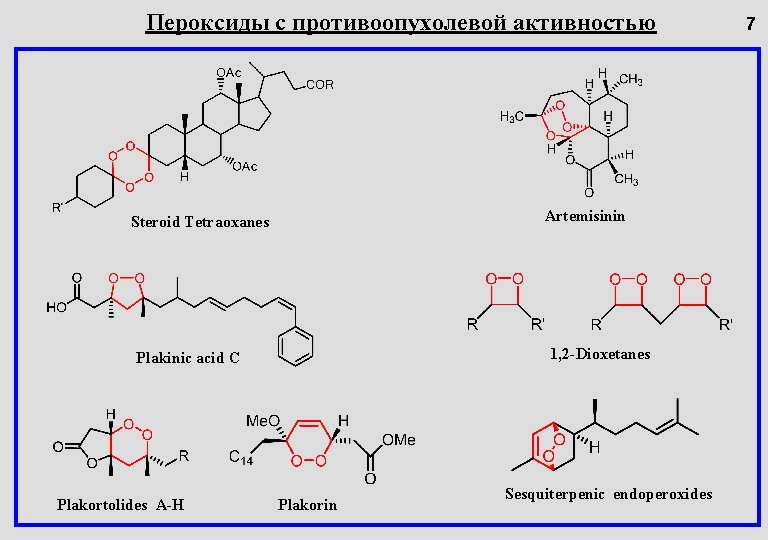
Пероксиды с противоопухолевой активностью Artemisinin Steroid Tetraoxanes 1, 2 -Dioxetanes Plakinic acid C Plakortolides A-H Plakorin Sesquiterpenic endoperoxides 7



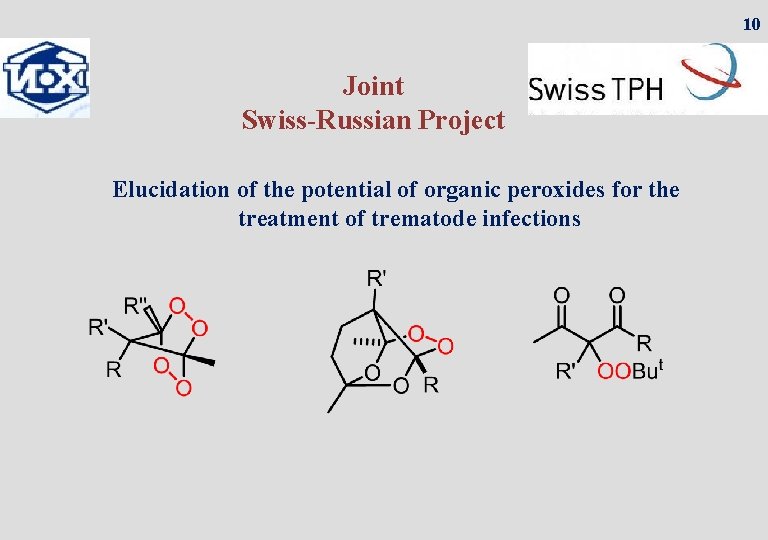
10 Joint Swiss-Russian Project Elucidation of the potential of organic peroxides for the treatment of trematode infections

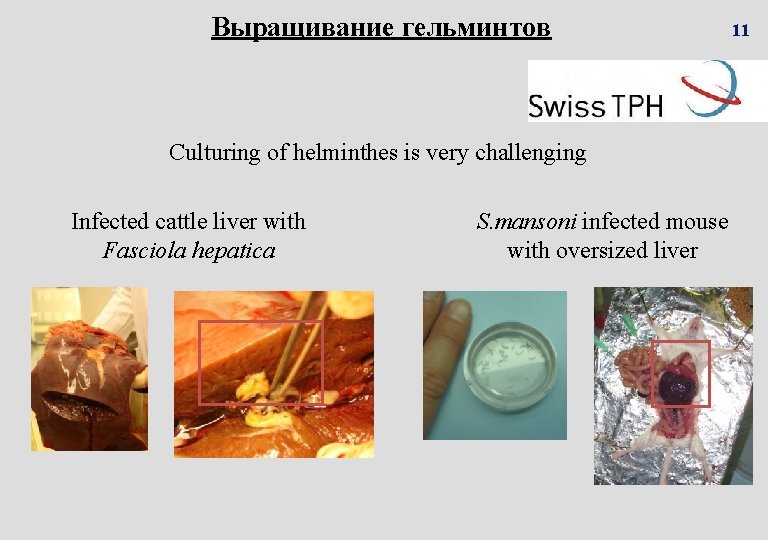
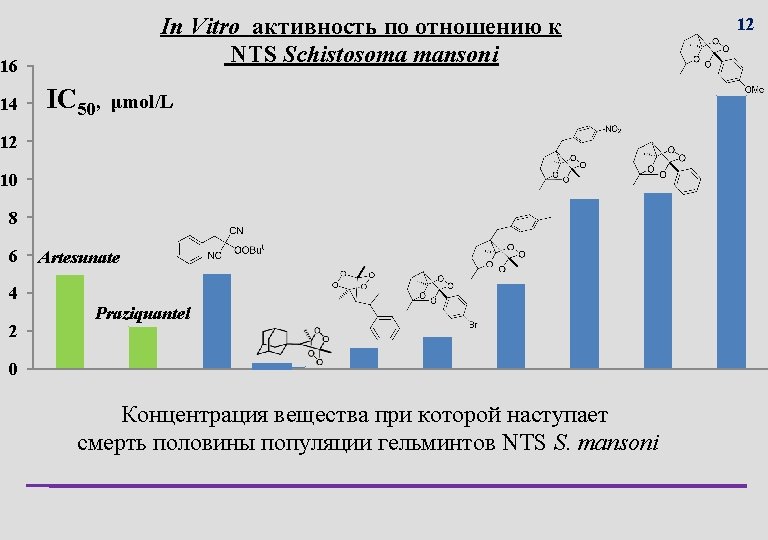
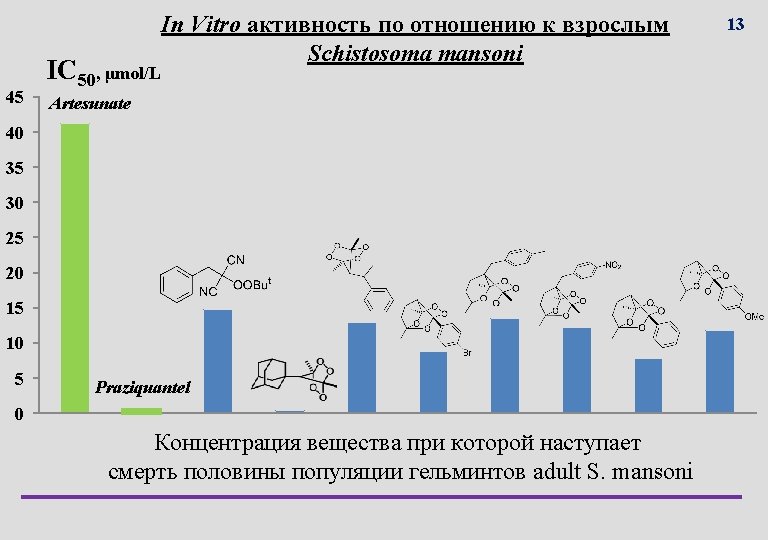
Выращивание гельминтов Culturing of helminthes is very challenging Infected cattle liver with Fasciola hepatica S. mansoni infected mouse with oversized liver 11



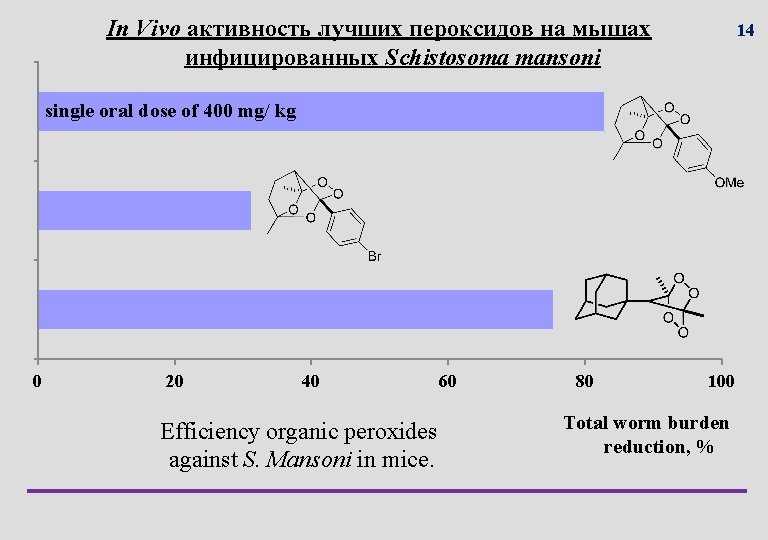
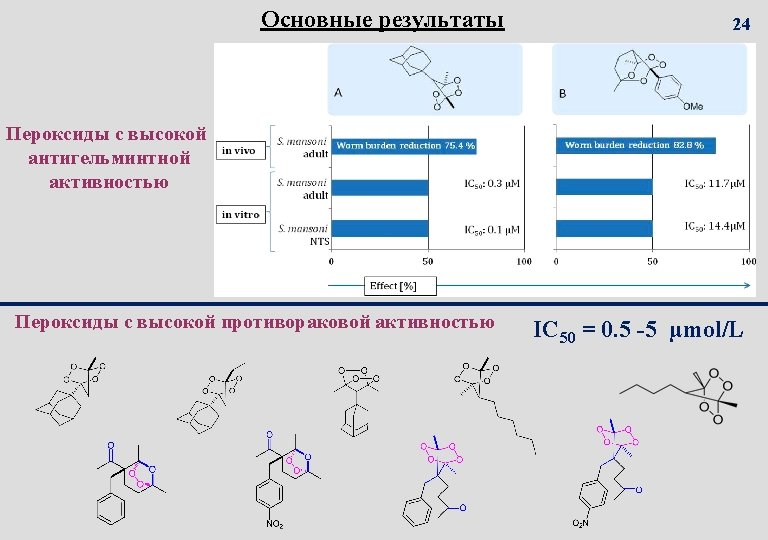
In Vivo активность лучших пероксидов на мышах инфицированных Schistosoma mansoni 14 single oral dose of 400 mg/ kg 0 20 40 Efficiency organic peroxides against S. Mansoni in mice. 60 80 100 Total worm burden reduction, %

15 Joint French-Russian Project Organic peroxide as potenial anticancer drugs

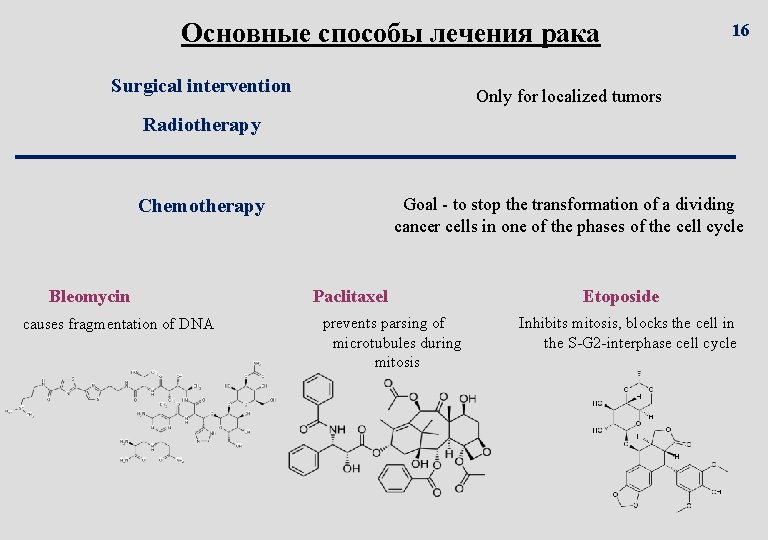
Основные способы лечения рака Surgical intervention 16 Only for localized tumors Radiotherapy Goal - to stop the transformation of a dividing cancer cells in one of the phases of the cell cycle Chemotherapy Bleomycin causes fragmentation of DNA Paclitaxel prevents parsing of microtubules during mitosis Etoposide Inhibits mitosis, blocks the cell in the S-G 2 -interphase cell cycle

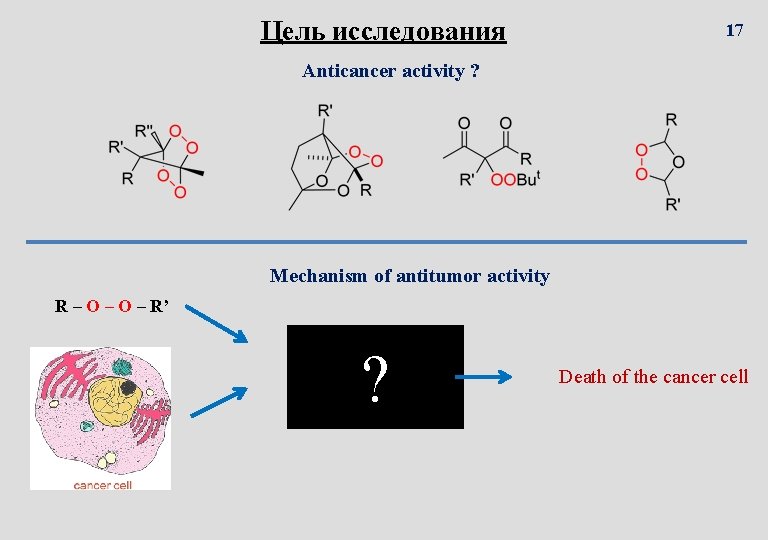
Цель исследования 17 Anticancer activity ? Mechanism of antitumor activity R – O – R’ ? Death of the cancer cell

Клеточная линия He. La cells Cervical cancer cells 18

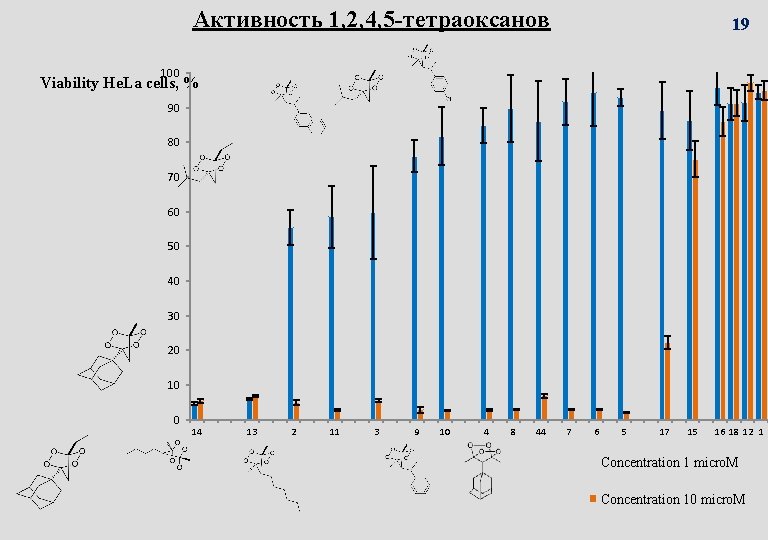
Активность 1, 2, 4, 5 -тетраоксанов 19 100 Viability He. La cells, % 90 80 70 60 50 40 30 20 10 0 14 13 2 11 3 9 10 4 8 44 7 6 5 17 15 16 18 12 1 Concentration 1 micro. M Concentration 10 micro. M

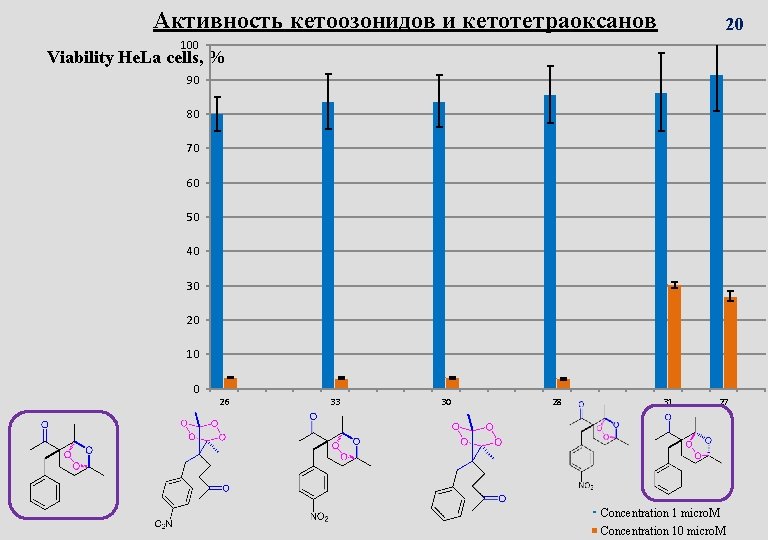
Активность кетоозонидов и кетотетраоксанов 20 100 Viability He. La cells, % 90 80 70 60 50 40 30 20 10 0 26 33 30 28 31 27 Concentration 1 micro. M Concentration 10 micro. M


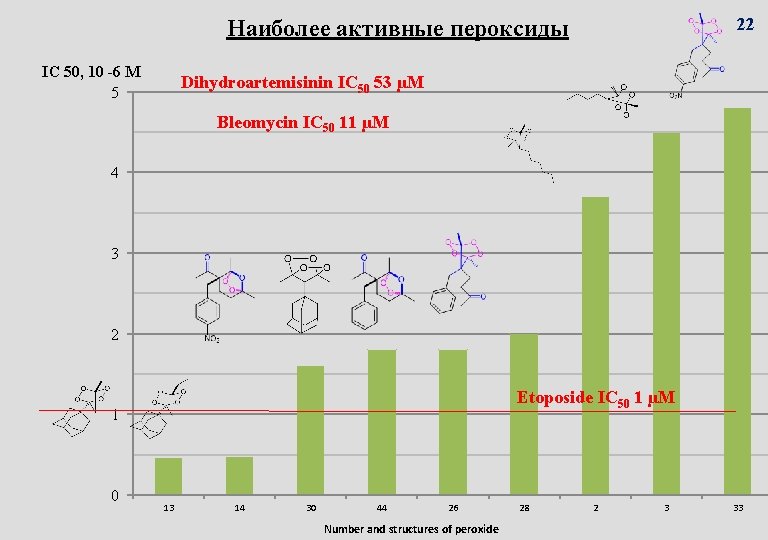
22 Наиболее активные пероксиды IC 50, 10 -6 M 5 Dihydroartemisinin IC 50 53 µM Bleomycin IC 50 11 µM 4 3 2 Etoposide IC 50 1 µM 1 0 13 14 30 44 26 Number and structures of peroxide 28 2 3 33

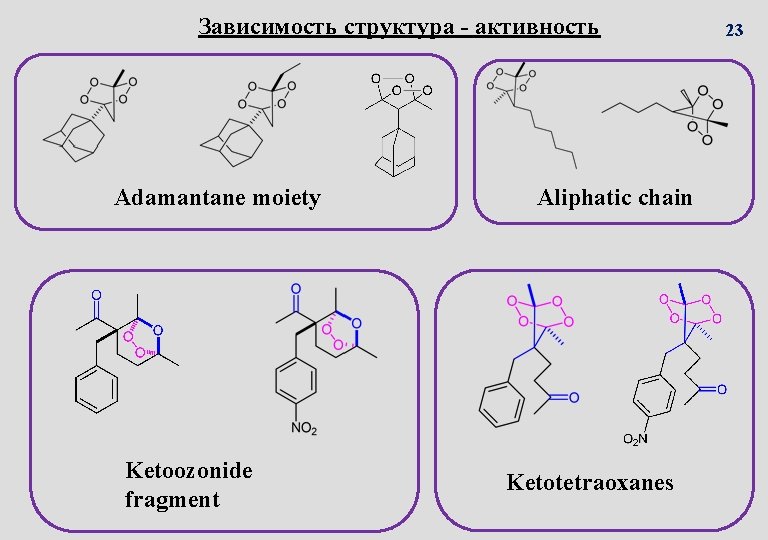
Зависимость структура - активность Adamantane moiety Ketoozonide fragment Aliphatic chain Ketotetraoxanes 23


Благодарность Министерству образования и науки Российской Федерации Swiss Tropical and Public Health Institute Prof. Jennifer Keiser Katrin Ingram Université de Nantes Prof. Dmitrii Olegovich Levitsky Prof. Fabrice Fleury 25

N. D. Zelinsky Institute of Organic Chemistry Russian Academy of Sciences Молодежная часть коллектива

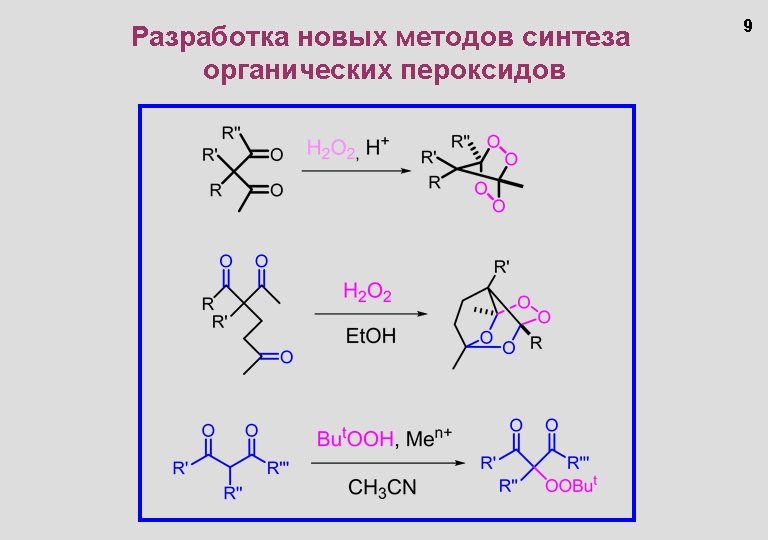
Основные публикации 1. Alexander O. Terent'ev, Dmitry A. Borisov, Ivan A. Yaremenko, Yuri N. Ogibin, Gennady I. Nikishin. Oxidation Of Substituted b-Diketones With Hydrogen Peroxide. Synthesis Of Esters Through The Formation Of Bridged 1, 2, 4, 5 -Tetraoxanes. // Synthesis, 2010, 1145 -1149. 2. Terent'ev Alexander; Borisov Dmitry; Yaremenko Ivan; Chernyshev Vladimir; Nikishin Gennady. Synthesis of Asymmetric Peroxides: Transition Metal (Cu, Fe, Mn, Co)-catalyzed Peroxidation of beta-Dicarbonyl Compounds with tert-Butyl Hydroperoxide. // J. Org. Chem. 2010, 75, 5065 -5071. 3. Терентьев А. О. , Кривых О. В. , Крылов И. Б. , Никишин Г. И. Способ получения цикличеcких геминальных бисгидропероксидов. // Заявка № 2010112293; 31. 03. 2010 (Application RU № 2010112293). Патент РФ № 2430087; 27. 09. 2011 Бюл. № 27 (Patent RU № 2430087). 4. М. Д. Веденяпина, А. О. Терентьев, М. М. Платонов, А. М. Скундин, А. А. Веденяпин, Г. И. Никишин. Электрохимическое окисление 1, 1 -дигидроперокси-4 -метилциклогексана на платиновом аноде. Синтез 3, 12 -диметил-7, 8, 15, 16 -тетраоксадиспиро[5. 2]гексадекана. // ЭЛЕКТРОХИМИЯ, 2011, том 47, № 2, с. 251– 254. 5. Alexander O. Terent’ev, Dmitry A. Borisov, Vsevolod V. Semenov, Vladimir V. Chernyshev, Valery M. Dembitsky, Gennady I. Nikishin. Selective Synthesis of Unsymmetrical Peroxides. Transition Metal (Cu, Fe, Mn, Co) Catalyzed Oxidation of Derivatives of Malononitrile and Cyanoacetic Ester by tert-Butyl Hydroperoxide at the α-Position. // Synthesis 2011, № 13, 2091 -2100. 6. А. О. Терентьев, М. М. Платонов, Д. О. Левицкий, В. М. Дембицкий. Органические пероксиды кремния и германия: синтез и реакции. // Успехи химии, 2011, Том 80, Номер 9, Страницы 843 -864 (2011). 7. Alexander O. Terent’ev, Ivan A. Yaremenko, Vladimir V. Chernyshev, Valery M. Dembitsky, Gennady I. Nikishin. Selective synthesis of cyclic peroxides from triketones and H 2 O 2. // J. Org. Chem. 2012, 77, 1833 -1842. 8. A. O. Terent’ev, D. A. Borisov, and I. A. Yaremenko. General methods for the preparation of 1, 2, 4, 5 -tetraoxanes – key structures for the development of peroxidic antimalarial agents. // Chemistry of Heterocyclic Compounds, 2012, Volume 48, Number 1, Pages 55 -58. 9. Терентьев А. О. , Ярёменко И. А. , Виль В. А. , Никишин Г. И. «Способ получения замещенных 2, 3, 5, 6 тетраоксабицикло[2. 2. 1]гептанов» . Заявка от 15. 08. 2012, регистрационный № 2012134730, входящий № 055589.
