OVERVIEW OF THE VIRGINIA MEDICAID PHARMACY PROGRAM Chethan










- Slides: 18

OVERVIEW OF THE VIRGINIA MEDICAID PHARMACY PROGRAM Chethan Bachireddy, MD, MSc Chief Medical Officer

Medicaid Drug Benefit q Optional benefit offered by all states q Defined by Social Security Act 1927 (the Act) § Medicaid programs are required to cover all drugs that are • FDA approved • Medically necessary • Manufactured by a pharmaceutical company participating in the Medicaid Drug Rebate Program § The Act allows the Medicaid program to develop preferred drug lists (PDLs) and exclude drugs from the PDL as long as a service authorization (SA) process is established 2

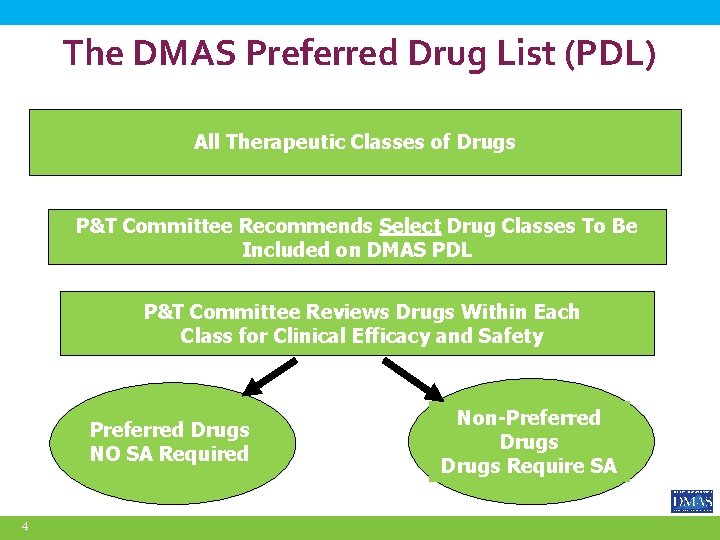
Preferred Drug List (PDL) Program q DMAS implemented a PDL in 2004 § Select drug classes are subject to the PDL program § Currently, there are 91 drug classes on the FFS PDL q Decisions regarding the PDL and service authorization criteria are made by DMAS’ Pharmacy and Therapeutics (P&T) Committee. § “Preferred” drugs are selected based on safety and clinical efficacy first, then on cost effectiveness § Supplemental rebates are collected on many but not all preferred drugs 3

The DMAS Preferred Drug List (PDL) All Therapeutic Classes of Drugs P&T Committee Recommends Select Drug Classes To Be Included on DMAS PDL P&T Committee Reviews Drugs Within Each Class for Clinical Efficacy and Safety Preferred Drugs NO SA Required 4 Non-Preferred Drugs Require SA

Medicaid Drug Rebates q Federal Rebates are collected on all FFS and MCO drug utilization q Supplemental Rebates are collected on select PDL preferred drugs § Negotiated “above and beyond” federal rebate q Both Federal and Supplemental Rebates are shared with the Federal government according to the State’s FMAP. q Over $800 million in drug rebates invoiced in FY 2020 5

DMAS Managed Care Organizations Provide Pharmacy Coverage q ~1. 6 million Virginians are covered by Medicaid q Approximately 95% of Medicaid members are enrolled with a managed care organization (MCO) q Six MCOs provide services for Medallion 4. 0 and CCC Plus members. q As part of the integrated delivery model, DMAS requires the health plans to utilize the Common Core Formulary. 6

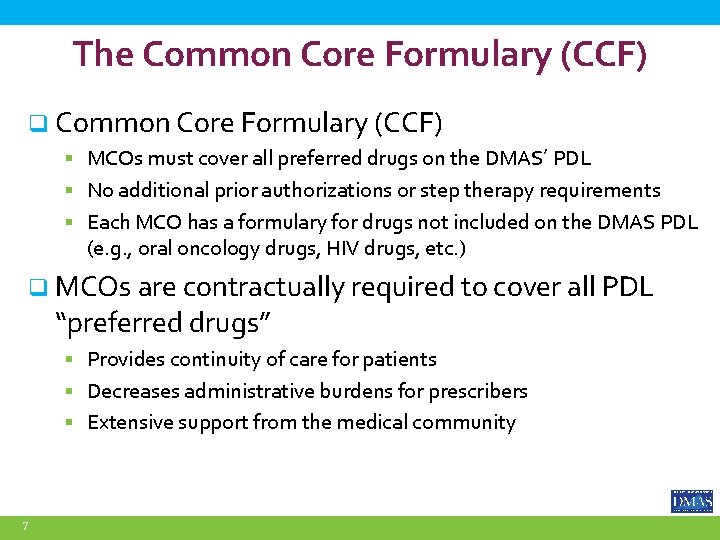
The Common Core Formulary (CCF) q Common Core Formulary (CCF) § MCOs must cover all preferred drugs on the DMAS’ PDL § No additional prior authorizations or step therapy requirements § Each MCO has a formulary for drugs not included on the DMAS PDL (e. g. , oral oncology drugs, HIV drugs, etc. ) q MCOs are contractually required to cover all PDL “preferred drugs” § Provides continuity of care for patients § Decreases administrative burdens for prescribers § Extensive support from the medical community 7

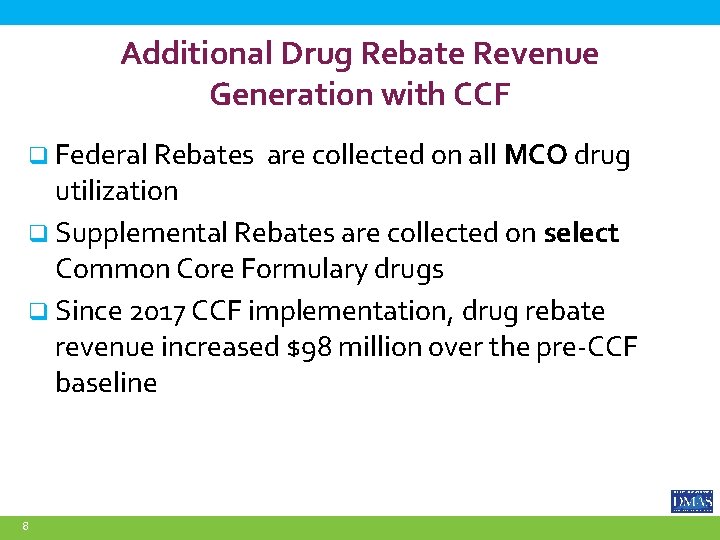
Additional Drug Rebate Revenue Generation with CCF q Federal Rebates are collected on all MCO drug utilization q Supplemental Rebates are collected on select Common Core Formulary drugs q Since 2017 CCF implementation, drug rebate revenue increased $98 million over the pre-CCF baseline 8

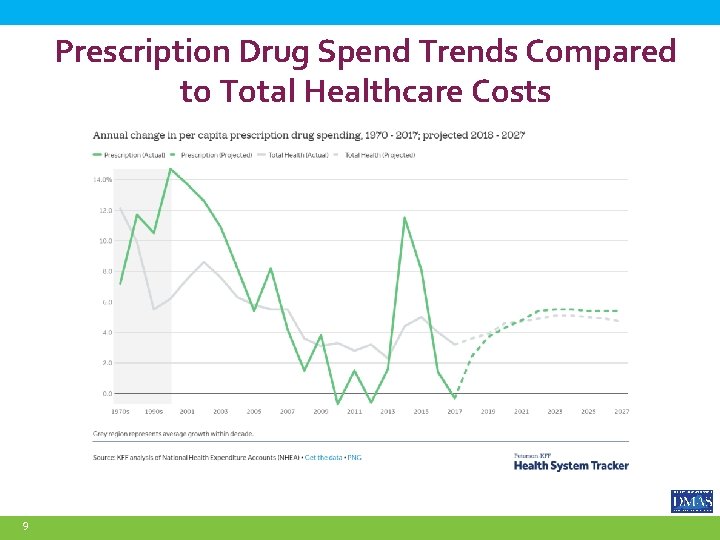
Prescription Drug Spend Trends Compared to Total Healthcare Costs 9

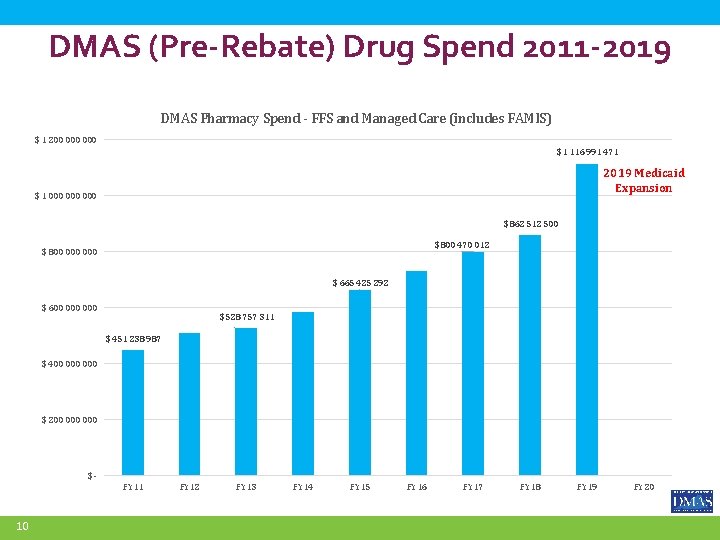
DMAS (Pre-Rebate) Drug Spend 2011 -2019 DMAS Pharmacy Spend - FFS and Managed Care (includes FAMIS) $ 1 200 000 $ 1 116 991 471 2019 Medicaid Expansion $ 1 000 000 $ 862 512 500 $ 800 470 012 $ 800 000 $ 665 425 292 $ 600 000 $ 528 757 311 $ 451 238 987 $ 400 000 $ 200 000 $FY 11 10 FY 12 FY 13 FY 14 FY 15 FY 16 FY 17 FY 18 FY 19 FY 20

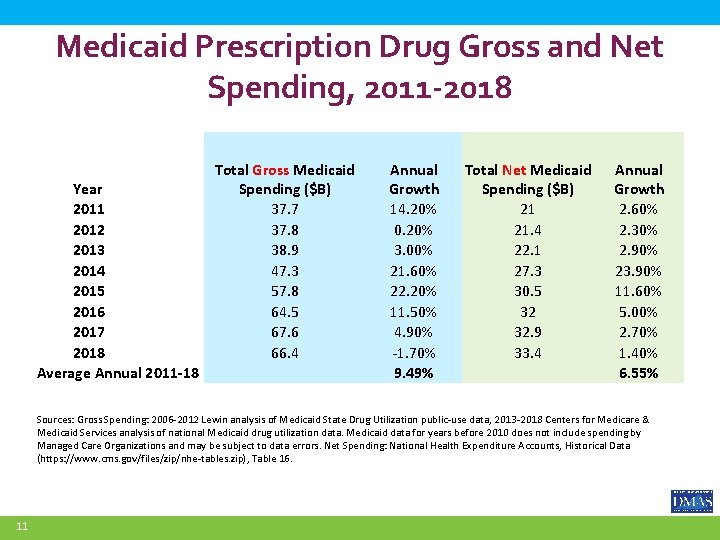
Medicaid Prescription Drug Gross and Net Spending, 2011 -2018 Year 2011 2012 2013 2014 2015 2016 2017 2018 Average Annual 2011 -18 Total Gross Medicaid Spending ($B) 37. 7 37. 8 38. 9 47. 3 57. 8 64. 5 67. 6 66. 4 Annual Growth 14. 20% 0. 20% 3. 00% 21. 60% 22. 20% 11. 50% 4. 90% -1. 70% 9. 49% Total Net Medicaid Spending ($B) 21 21. 4 22. 1 27. 3 30. 5 32 32. 9 33. 4 Annual Growth 2. 60% 2. 30% 2. 90% 23. 90% 11. 60% 5. 00% 2. 70% 1. 40% 6. 55% Sources: Gross Spending: 2006 -2012 Lewin analysis of Medicaid State Drug Utilization public-use data, 2013 -2018 Centers for Medicare & Medicaid Services analysis of national Medicaid drug utilization data. Medicaid data for years before 2010 does not include spending by Managed Care Organizations and may be subject to data errors. Net Spending: National Health Expenditure Accounts, Historical Data (https: //www. cms. gov/files/zip/nhe-tables. zip), Table 16. 11

Strategies to Address Escalating Drug Spend q DMAS PDL & Common Core Formulary q Cost-Effective Pharmacy Benefit Delivery Models q Specialty Drug Management q Innovative Drug Coverage & Financing Reforms 12

Cost-Effective Pharmacy Benefit Delivery Model Evaluation q 2019 GA Report to evaluate and determine the most cost-effective pharmacy benefit delivery model. § Carve-in vs Carve-out § Spread vs Pass-through reimbursement by Pharmacy Benefit Managers (PBMs) contracted by MCOs § MCO Pharmacy Reimbursement Aligned with FFS q 2020 GA directed DMAS to prohibit spread pricing by MCO contracted PBMs. § Effective 7/1/2020, all six managed care PBMs are mandated to use “pass-through” pricing 13

High-Cost Specialty Drugs Specialty drugs are drugs that require special handling, administration or monitoring and are used to treat complex, chronic and often costly conditions (e. g. multiple sclerosis, rheumatoid arthritis, hepatitis C, and hemophilia). q Prescription drug costs are the fastest growing segment of US healthcare spending. q These costs are growing in part due to increased market entry of extremely high-cost break-through drugs. Some recent examples include: q § Zolgensma, a $2. 1 million gene therapy for Spinal Muscular Atrophy § Luxturna, an $850 K gene therapy for Leber’s Congenital Amaurosis (congenital blindness) § Trikafta, a $300 K therapy for Cystic Fibrosis 14

Virginia’s 2021 -22 Budget Item 313. CCCCC Directs DMAS to develop a process for the determination of Medicaid coverage and reimbursement of FDA fast-track drugs and emerging-break-through technologies. q Including: q § (1) a determination of whether Virginia Medicaid will cover the drug or technology; § (2) upon determination of coverage, a determination of uniform clinical criteria for coverage; § (3) upon determination of clinical criteria for coverage, mandated application of the clinical criteria for coverage across Fee-For-Service and Managed Care Organizations; and § (4) the development of an actuarially-sound reimbursement methodology for MCOs to include kick-payments or other passthrough arrangements consistent with the utilization and cost of the drug or technology. 15

Innovative Drug Coverage & Financing Reforms q DMAS partnership with the Center for Evidence- Based Policy § DERP (Drug Effectiveness Review Project) / MED (Medicaid Evidence-based Decisions) Project • a collaborative of state Medicaid and public pharmacy programs that produces concise, comparative, evidencebased reports to assist state policymakers with decisions for improving health outcomes. § Smart-D (State Medicaid Alternative Reimbursement and Purchasing Test for High-Cost Drugs) • purpose is to help bring clarity to the complicated landscape of drug purchasing for state Medicaid programs to help improve patient access to clinically effective therapies while assisting state Medicaid programs with alternative payment models. 16

Recommendations for Managing Escalating Drug Costs q Continue to evaluate and improve the effectiveness of the Common Core Formulary q Support drug pricing and PBM reimbursement transparency policies q Explore value based purchasing arrangements with manufacturers 17

Questions? 18