Overview of the EU regulatory system and governance

















- Slides: 20

Overview of the EU regulatory system and governance PANDRH Conference – 5 September 2013 Ottawa Presented by: Alexios Skarlatos European Medicines Agency – Head of Product Information Quality An agency of the European Union

CONTENTS 1. Approval of medicines in the EU 2. EU assessment collaboration: Organisational aspects 3. EU assessment collaboration: Enablers and facilitators 2

1. Approval of medicines in EU 3

A medicinal product may only be placed on the market in the EU, when a marketing authorisation has been issued

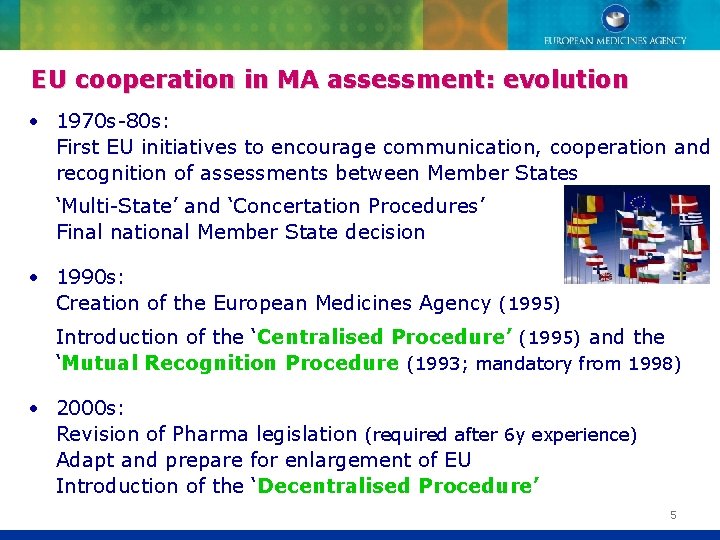
EU cooperation in MA assessment: evolution • 1970 s-80 s: First EU initiatives to encourage communication, cooperation and recognition of assessments between Member States ‘Multi-State’ and ‘Concertation Procedures’ Final national Member State decision • 1990 s: Creation of the European Medicines Agency (1995) Introduction of the ‘Centralised Procedure’ (1995) and the ‘Mutual Recognition Procedure (1993; mandatory from 1998) • 2000 s: Revision of Pharma legislation (required after 6 y experience) Adapt and prepare for enlargement of EU Introduction of the ‘Decentralised Procedure’ 5

Drivers of Harmonisation • The global pharmaceutical market -> effective operation of the internal market • Pharmaceutical industry -> facilitate the free movement of medicines • Scientific culture • Patient involvement • Need for worksharing -> avoid unnecessary duplication of efforts • Fear of the unknown - insecurity 6

Obstacles to harmonisation • Giving up sovereignty • Need for compromising • Heterogeneous cultures • Language issue • Uneven levels of capacity/experience • Uneven share of the workload 7

Current Marketing Authorisation procedures in EU Approval in 1 MS => National Authorisation procedure Approval in n or all MSs => EU Authorisation procedure 3 EU procedures: Centralised Procedure (CP) 1 single EU MA Mutual Recognition Procedure (MRP) Decentralised Procedure (DCP) n national MAs Route? Choice? Depending on type of product and authorisation history in EU + Regulatory & marketing strategy Company preferences etc … 8

Eligibility to the Centralised Procedure “Mandatory Scope” - Biotech products - New Active Substances for Cancer, Diabetes, AIDS, Neurodegenerative, viral and auto-immune diseases - Orphan medicinal products - Cell & gene therapy + tissue engineered products “Optional Scope” - Other new active substances - Innovative uses of ‘old’ substances - Interest of patients at EU level - Generics of centrally-authorised products 9

2. EU assessment collaboration: Organisational aspects 10

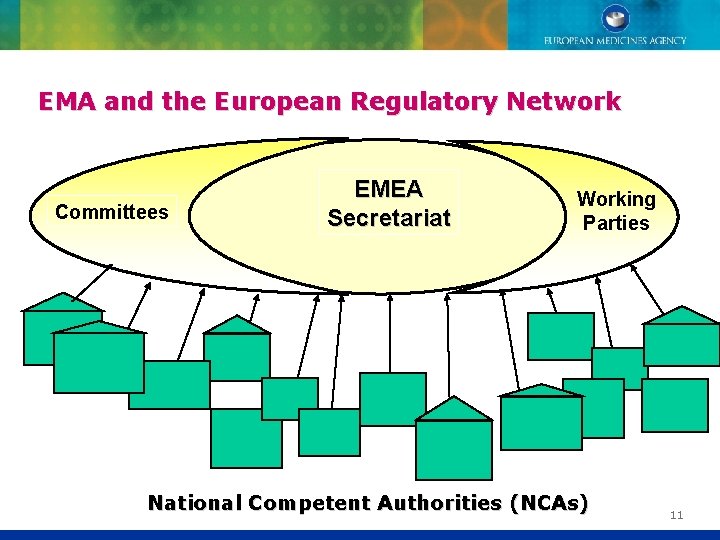
EMA and the European Regulatory Network Committees EMEA Secretariat Working Parties National Competent Authorities (NCAs) 11

Resources in the Centralised Procedure • National Competent Authorities (NCAs) in 28 EU Member States. EMA not intended to replace NCAs, but to be a trusted partner in the EU Regulatory Network • > 3500 scientific experts nominated by Member States Available for scientific work & assessments for EMA • Scientific committee (CHMP) delivers a final opinion • Multidisciplinary assessment teams in MS to perform assessment (as Rapporteur/Co-Rapporteur) or participate as ‘peer reviewer’ • Services are provided to EMA on the basis of a cooperation agreement • All agencies linked by secure IT network (Eudra. Net) • Fees received by EMA payment of (Co-)Rapporteur 12

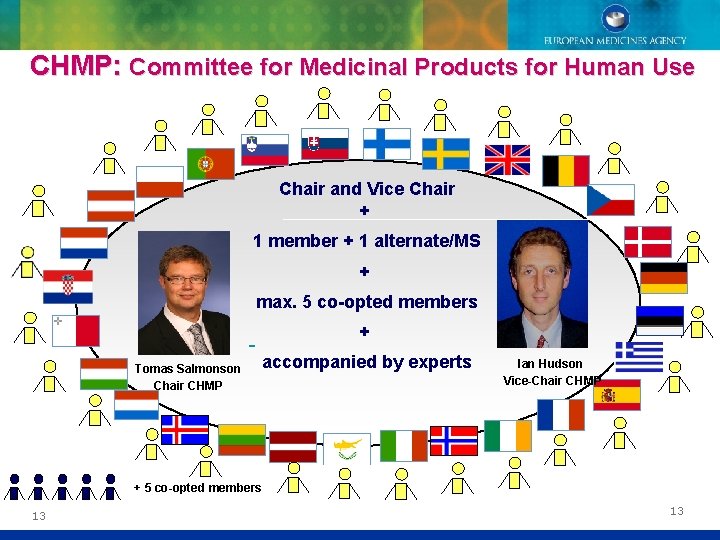
CHMP: Committee for Medicinal Products for Human Use Chair and Vice Chair + 1 member + 1 alternate/MS + max. 5 co-opted members + Tomas Salmonson Chair CHMP accompanied by experts Ian Hudson Vice-Chair CHMP + 5 co-opted members 13 13

Support for MRP & DCP: Coordination group • Coordination Group for Mutual Recognition and Decentralised Procedures – human (CMDh) • Initially, voluntary ‘Mutual Recognition Facilitation Group’ • Recognised and legal mandate in EU legislation revision • Representatives of all Member States • Agree on common rules procedural / regulatory matters Guidance to applicants • Discuss and solve scientific issues during MRP/DCP • EMA: secretariat, organisation of monthly meetings at EMA 14

3. EU assessment collaboration: enablers & facilitators 15

• Harmonised legal requirements (high level) for Marketing Authorisation Application • Harmonisation of technical standards and requirements ICH guidelines, incl. e. CTD CHMP scientific guidelines - applicable to CP, MRP, DCP • Provision of regulatory guidance Agreement on common approaches & procedures Commission guidelines CHMP and EMA guidance documents, Q&As, SOPs Advice to applicants Consistent approach/interpretation between Member States 16

• Working together in expert Working Parties or ad-hoc groups e. g. Quality Working Party GCP inspectors working group • Training of assessors • Common templates for assessment reports • ‘Twinning’ projects, e. g. for new EU Member States • Years of confidence building, working together, worksharing • Oversight and management by ‘Heads of Medicines Agencies’ (HMA) Group • Secure communication tools and databases 17

Member State cooperation in MRP and DCP • «Coordination Group for Mutual Recognition and Decentralised Procedures» CMDh, since Nov 2005 Replaces the informal MRFG group Coordination and facilitation of the operation of MRP and DCP Discussions on procedural/regulatory issues, guidance docs + scientific discussions on applications where MSs disagree Ensure consistency of standards and good decision-making Avoid referrals to EMA 18

• Disagreements in MRP and DCP: only on the basis of ‘potential serious risk to public health’ Commission Guideline: definition and examples CMDh: ensure common understanding and application • Harmonisation of national Sm. PCs of originators, in order to facilitate approval of generics accross EU • Commission guidelines on MRP and DCP procedures + CMDh publication of ‘Best Practice Guides’ Procedural guidance documents Q&A • Worksharing and working group initiatives, coordinated by CMDh e. g. on PSURs and Active Substance Master Files 19

Thank you Alexios M. Skarlatos Head of Product Information Quality + 44 207 418 8682 alexios. skarlatos@ema. europa. eu 20