Overview of the Continuity of Care Record Claudia




































- Slides: 64

Overview of the Continuity of Care Record Claudia Tessier, CAE, RHIA Co-Chair, ASTM E 31 CCR Workgroup Executive Director, Mo. HCA

The CCR: A Snapshot in Time n A core data set of the most relevant current and past information about a patient’s health status and healthcare treatment n Organized and transportable n Prepared by a practitioner at the conclusion of a healthcare encounter n Enables the next practitioner to readily access such information

Unique Standards Development Effort n Consortium of sponsoring organizations n n n n n ASTM International E 31 Committee on Health Informatics Massachusetts Medical Society HIMSS American Academy of Family Physicians American Academy of Pediatrics American Medical Association Patient Safety Institute American Health Care Association National Association for the Support of LTC Mobile Healthcare Alliance (Mo. HCA)

Sponsors represent… n ANSI-recognized standards development organization n Over 400, 000 practitioners n Over 13, 000 IT professionals n Over 12, 000 institutions in the long-term care community providing care to over 1. 5 million elderly and disabled n Major stakeholders in m-Health n Patients, patient advocates, data sources, corporations, provider institutions….

What About HL 7? n ASTM and HL 7: memorandum of understanding to harmonize n n ASTM’s CCR and HL 7’s EHR functionality, CDA, and RIM standards n Work is ongoing to achieve that aim

This Unique Initiative Is… n Patient-focused n Not about what the system says to do but about what patient information is most relevant n Provider-focused n Practitioners determine what information is most relevant to the next provider in order to deliver good patient care

What’s in the CCR’s Core Data Set? n CCR Header n CCR Body n CCR Footer

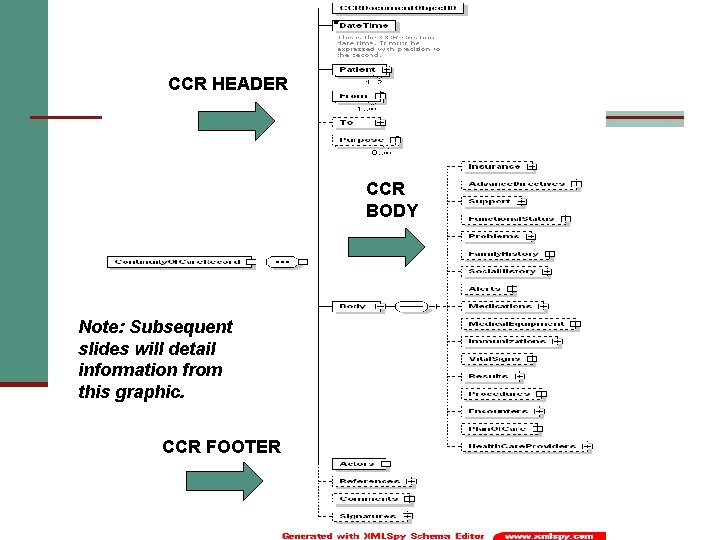
CCR HEADER CCR BODY Note: Subsequent slides will detail information from this graphic. CCR FOOTER

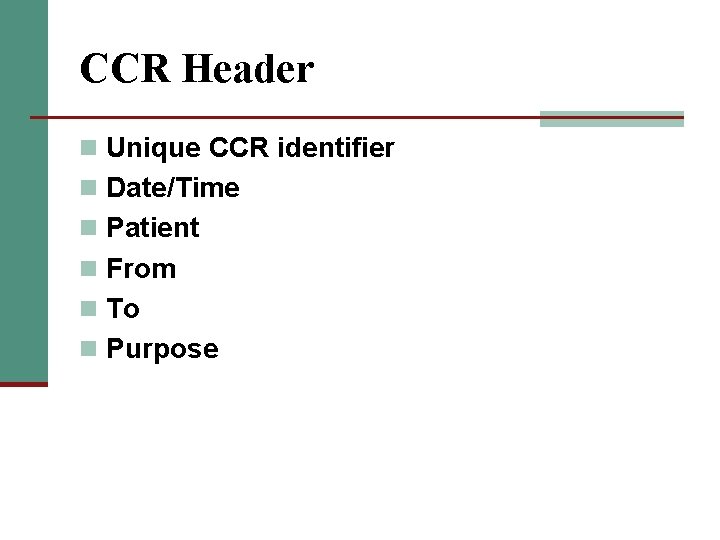
CCR Header n Unique CCR identifier n Date/Time n Patient n From n To n Purpose

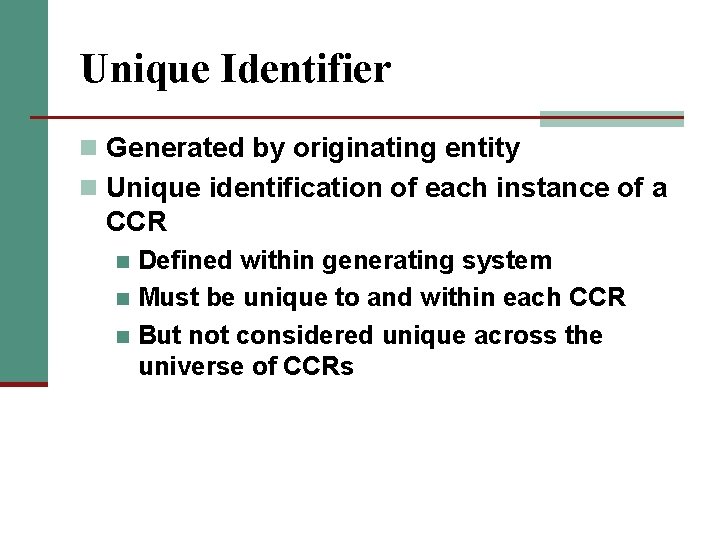
Unique Identifier n Generated by originating entity n Unique identification of each instance of a CCR Defined within generating system n Must be unique to and within each CCR n But not considered unique across the universe of CCRs n

Date/Time n Exact clock time that specific CCR was created/generated

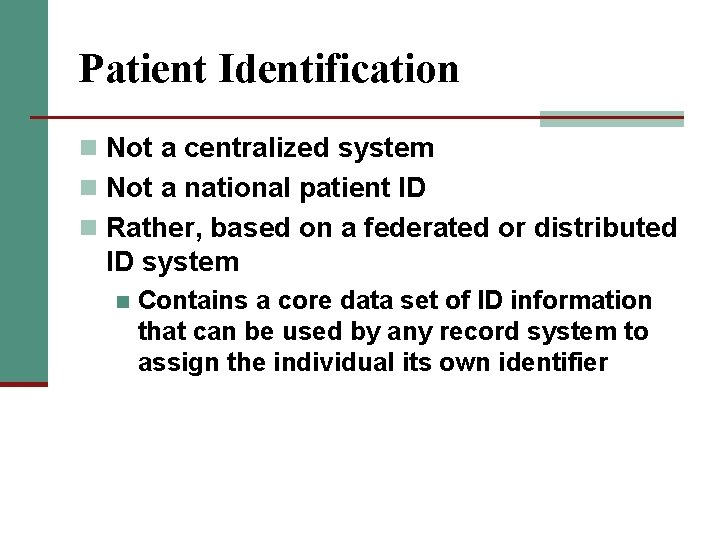
Patient Identification n Not a centralized system n Not a national patient ID n Rather, based on a federated or distributed ID system n Contains a core data set of ID information that can be used by any record system to assign the individual its own identifier

From n Identifies practitioner, person, system, or organization that generated the CCR n Also defines the healthcare role that each entity is playing when generating the CCR

To n Identifies the intended recipient/s of CCR n Practitioner, person, system, or organization

Purpose n The reason the CCR was created, e. g. , n Referral n Transfer n Discharge n Personal health record n Other….

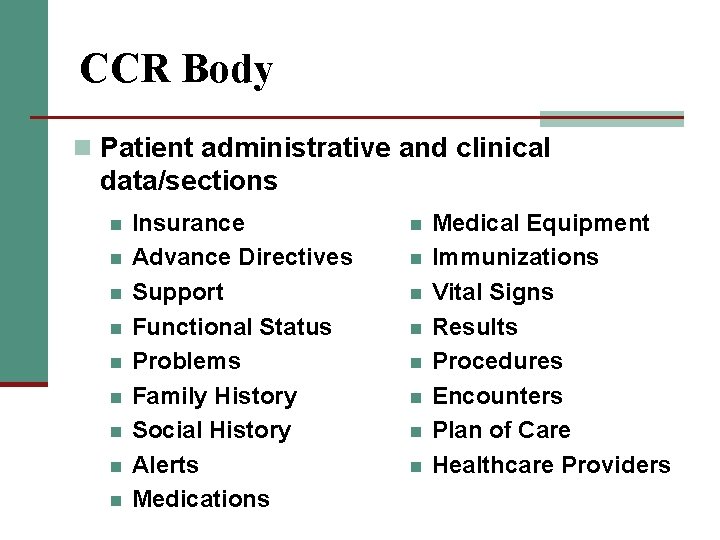
CCR Body n Patient administrative and clinical data/sections n n n n n Insurance Advance Directives Support Functional Status Problems Family History Social History Alerts Medications n n n n Medical Equipment Immunizations Vital Signs Results Procedures Encounters Plan of Care Healthcare Providers

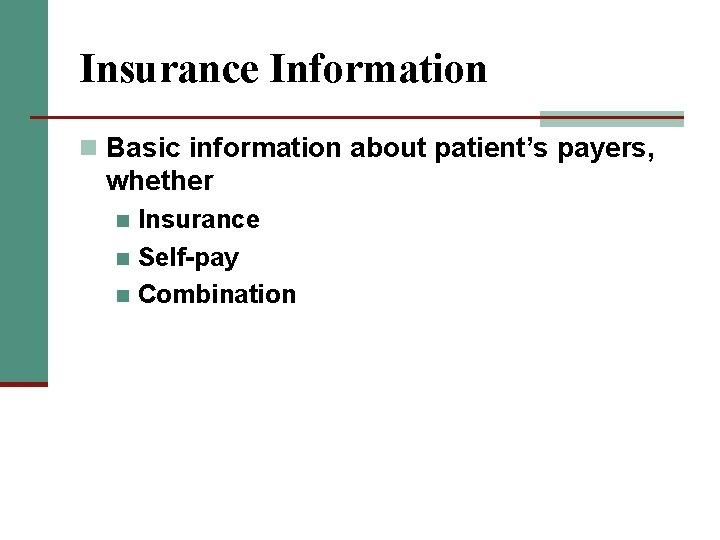
Insurance Information n Basic information about patient’s payers, whether Insurance n Self-pay n Combination n

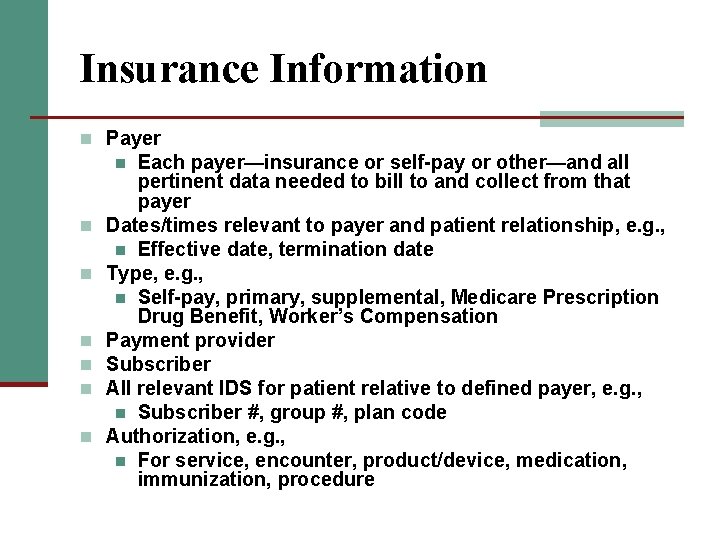
Insurance Information n Payer Each payer—insurance or self-pay or other—and all pertinent data needed to bill to and collect from that payer Dates/times relevant to payer and patient relationship, e. g. , n Effective date, termination date Type, e. g. , n Self-pay, primary, supplemental, Medicare Prescription Drug Benefit, Worker’s Compensation Payment provider Subscriber All relevant IDS for patient relative to defined payer, e. g. , n Subscriber #, group #, plan code Authorization, e. g. , n For service, encounter, product/device, medication, immunization, procedure n n n n

Advance Directives n Itemizes specific requests of patient and family regarding clinical interventions and specific resuscitation efforts to be undertaken in event of specific clinical outcomes or complications n Which are to be restricted, limited, or avoided as addressed in such documents as n n Living wills Healthcare proxies Powers of attorney for healthcare If none or unknown, this must be stated

Support n Lists patient’s sources of support, e. g. , n Immediate family n Relatives n Guardian n Durable power of attorney for healthcare n Spiritual advisor/clergy n Individuals or organizations n Not healthcare providers, which are identified in another section

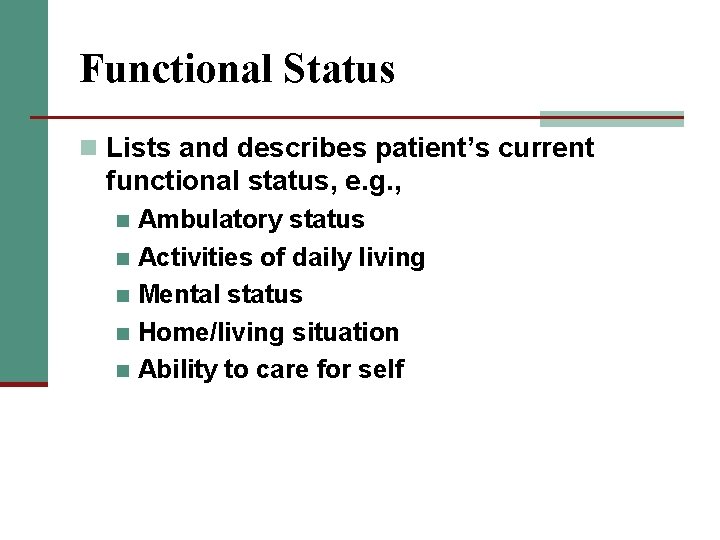
Functional Status n Lists and describes patient’s current functional status, e. g. , Ambulatory status n Activities of daily living n Mental status n Home/living situation n Ability to care for self n

Problems n Lists and describes all relevant clinical conditions, diagnoses, and problems For referrals, in order of importance n Otherwise, reverse chronological order of onset is preferred n

Family History n Identifies the health or health risk of a patient relative to health conditions seen in the family, including that family member’s Relationship to patient n Problem n Status n Other relevant data n

Social History n Information on social history, including n n n Marital status Religion Ethnicity Race Language Smoking n n n Diet Exercise Employment Toxic exposure ETOH use Drug use

Alerts n Lists and describes any of the following that are pertinent to patient’s current or past medical history Allergies n Adverse drug reactions (ADR) n Alerts n

Medications n Lists relevant current and past medications prescribed and administered n n n n n Brand generic names Dose strength and units Form or presentation Quantity, route, frequency Directions Refills Fulfillment Current status And more n Also OTC medications, vitamins, etc. n Can be linked to problems and to practitioners

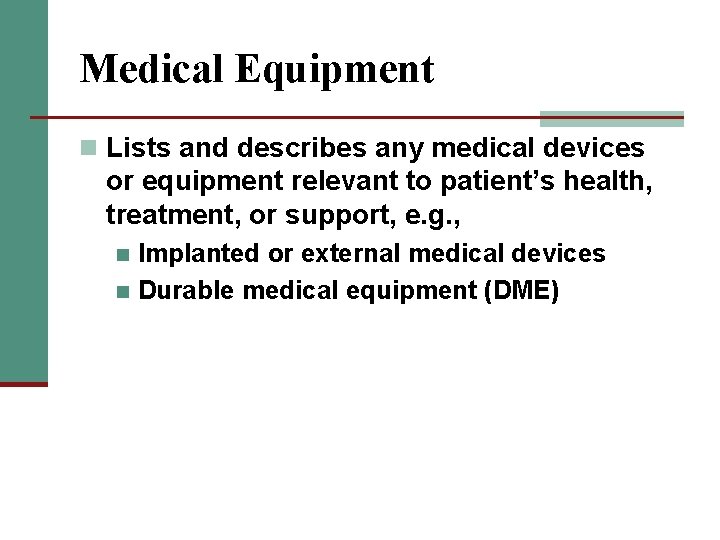
Medical Equipment n Lists and describes any medical devices or equipment relevant to patient’s health, treatment, or support, e. g. , Implanted or external medical devices n Durable medical equipment (DME) n

Immunizations n Lists and describes immunizations n Recently received or n Pertinent to patient’s health history

Vital Signs n Includes pertinent vital signs, e. g. , n Blood pressure n Pulse n Respiratory rate n Height n Weight n Body mass index n Head circumferences n Crown-to-rump length n Pulse oximetry n Pulmonary function tests

Results n Captures detailed laboratory, diagnostic, and therapeutic results data n Includes such information as Test or observation n Data/time sample obtained n Substance n Test type n Value and units n Method n Status n And more n

Procedures n Lists and describes any diagnostic and/or therapeutic procedures pertinent to the patient’s current health status or relevant past history, e. g. , Cardiac cath, x-ray, etc. n CABG, chemotherapy, etc. n Health status assessments, e. g. , n n Functional assessments Ambulatory status Suicide risk assessment

Encounters n Lists and describes any healthcare encounters pertinent to the patient’s current health status or relevant health history, including Hospitalizations n Office or clinic visits n Emergency room visits n Home health visits n Any other relevant treatment or therapy n

Plan of Care n Lists and describes any active, incomplete, or pending events of clinical significance to the current and ongoing care of the patient, including Orders n Appointments n Referrals n Procedures n Services n

Healthcare Providers n Includes information about all those healthcare providers who are participants in the patient’s care, e. g. , Primary physician n Any active consultants, clinicians, therapists, counselors n

CCR Footer n Actors n References n Comments n Signatures

Actors n Includes all detailed identifying information about each person, organization, location, or system referred to within the CCR, including the Patient

References n Lists the details concerning all references within the CCR to external data sources, e. g. , Living will n Durable power of attorney for healthcare n

Comments n Contains all comments referenced within the CCR n Free text only n Not for data that correctly belongs under other appropriate explicit fields/tags

Signatures n Contains all digital signatures relevant to the CCR

Annex A: Data Groups and Data Fields n A spreadsheet providing detailed list of CCR data groups and data elements within the CCR header, body, and footer, e. g. , n n Problems Medications

Data Groups n In addition to data elements specific to its purpose, each data group in the CCR Body and Footer also includes n Data source n n Internal CCR link n n Defines internal CCR links, e. g. , Problem to Healthcare Provider Comment n n Who or what is the source of the information Any relevant information that doesn’t fit elsewhere Reference n n Pointer to another data source or document that provides more information, e. g. living will, images. May include location where it can be found

Data Fields n Detailed information is provided for all data fields within each data group, including n n n XML code Definition Explanations, descriptions, requirements, and restrictions Comments and examples Specification of whether the field is required or optional

Annex B: XML Schema (. xsd) n Derived from XML codes in Annex A n Represents how the CCR should be represented in XML

Annex C: Implementation Guide (IG) n Instructions for using the CCR XML. xsd (in Annex B) for generation of a standardscompliant, interoperable CCR n Extremely strict regarding Requirements on use and formatting of the CCR XML n Content allowed within each field/XML tag n n The. xsd (see Annex B) must be used with the IG for validation of a CCR

XML Schema (. xsd) and Implementation Guide (IG) n Strict adherence to. xsd and IG is required when preparing CCR in structured electronic format n n To support standards-compliant interoperability To enable CCR to be prepared, transmitted, and viewed n n n In a browser In an HL 7 CDA-compliant document In secure email In any XML-enabled word processing document In multiple formats To enable properly designed EHR systems to n n n Import and export all CCR data Interchange the CCR between otherwise incompatible systems Minimize workflow disruption for practitioners

Coding n Detailed coding is recommended whenever practical within the CCR n The coding system and version must be specified n Coding systems are identified for Problems n Procedures n Products and agents n Results n

Coding Problems n Code at highest level using most recent pertinent national or international reimbursement codes at time CCR is generated, ICD-9 CM codes in US, for example n In addition, code with SNOMED CT codes to as granular a level as possible to support reporting, data analysis, and decision support

Coding Procedures n Code at highest level using most recent pertinent national or international reimbursement codes at time CCR is generated, e. g. , CPT codes in the US n In addition, code with LOINC codes to as granular a level as possible to support order entry, results reporting, data analysis, and decision support,

Coding Products and Agents n Code with appropriate products codes (such as Rx. Norm for medications in the US) to as granular a level as possible n In addition, may code with another standard as applicable (e. g. , NDC) or proprietary (drug information database) code with the type of code and source and version clearly defined.

Coding Results n Code with the most recent and appropriate result codes at the time the CCR is generated, e. g. , in the US CPT and LOINC for Procedures n LOINC for Result and Test in the US n

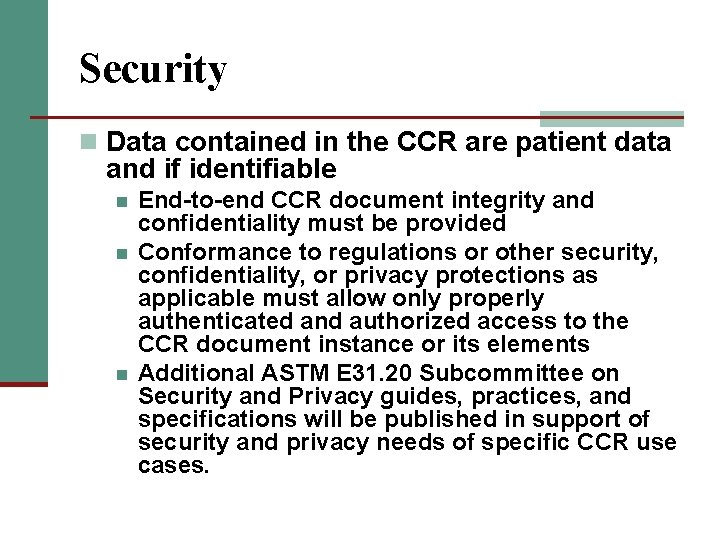
Security n Data contained in the CCR are patient data and if identifiable n n n End-to-end CCR document integrity and confidentiality must be provided Conformance to regulations or other security, confidentiality, or privacy protections as applicable must allow only properly authenticated and authorized access to the CCR document instance or its elements Additional ASTM E 31. 20 Subcommittee on Security and Privacy guides, practices, and specifications will be published in support of security and privacy needs of specific CCR use cases.

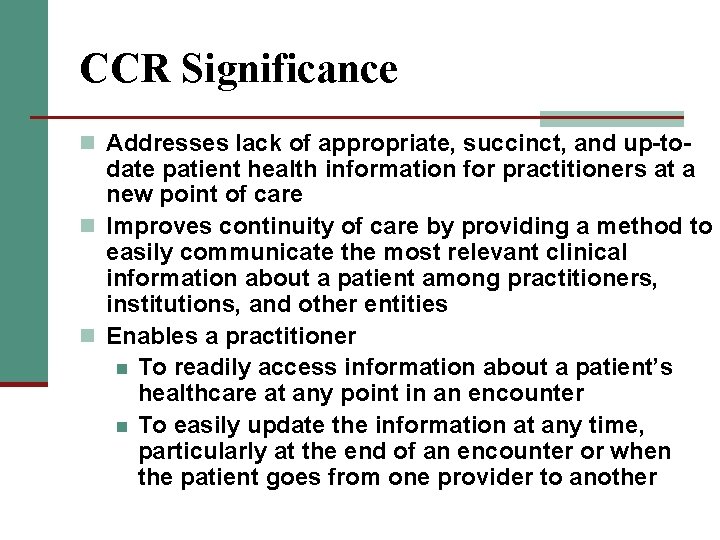
CCR Significance n Addresses lack of appropriate, succinct, and up-to- date patient health information for practitioners at a new point of care n Improves continuity of care by providing a method to easily communicate the most relevant clinical information about a patient among practitioners, institutions, and other entities n Enables a practitioner n To readily access information about a patient’s healthcare at any point in an encounter n To easily update the information at any time, particularly at the end of an encounter or when the patient goes from one provider to another

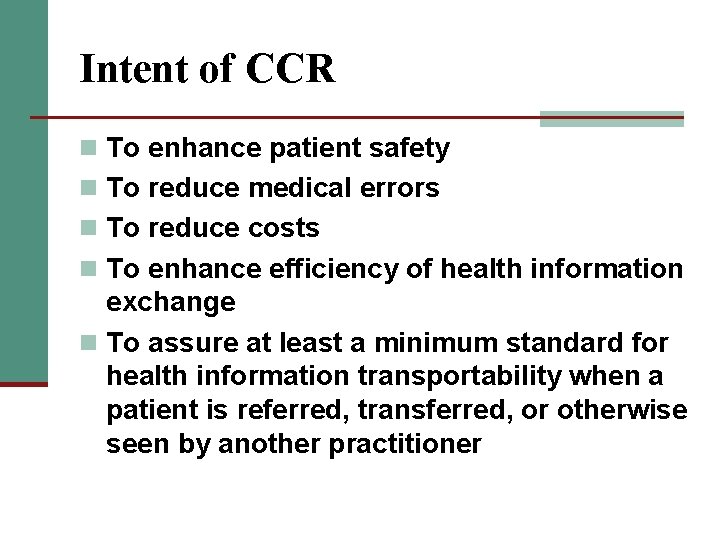
Intent of CCR n To enhance patient safety n To reduce medical errors n To reduce costs n To enhance efficiency of health information exchange n To assure at least a minimum standard for health information transportability when a patient is referred, transferred, or otherwise seen by another practitioner

Who Will Use the CCR and When? n The CCR will be completed by providers, e. g. , physicians, nurses, and ancillary practitioners, for Referral (inpatient or outpatient) n Transfer (from an inpatient or institutional setting) n Discharge without a referral or transfer n Personal health record n Other uses, e. g. , home health monitoring, school health, public health reporting n

Potential Domain-specific Applications n Enterprise- and institution-specific information Hospital to nursing and rehab facilities or home care agencies, and vice versa n Disease management-specific information, e. g. , n Diabetes, congestive heart failure, asthma, etc. n May be utilized by health plans, pharmaceutical companies, patient advocacy groups, others interested in promoting “best practices” n Payer-related information, e. g. , claims attachments n Patient-entered personal health information n

The CCR… n Is an introduction to electronic documentation and the EHR n Accommodates any relevant patient information, on paper or electronically n Supports patient safety and reduced medical errors n Easy access to critical data, e. g. , allergies n Has potential to reduce inefficiencies and costs n Don’t have to search for relevant information n Fewer repeat lab tests and other evaluations n Is not a top-down approach n n End-users, i. e. , practitioners have participated in its design Originator determines the relevant content

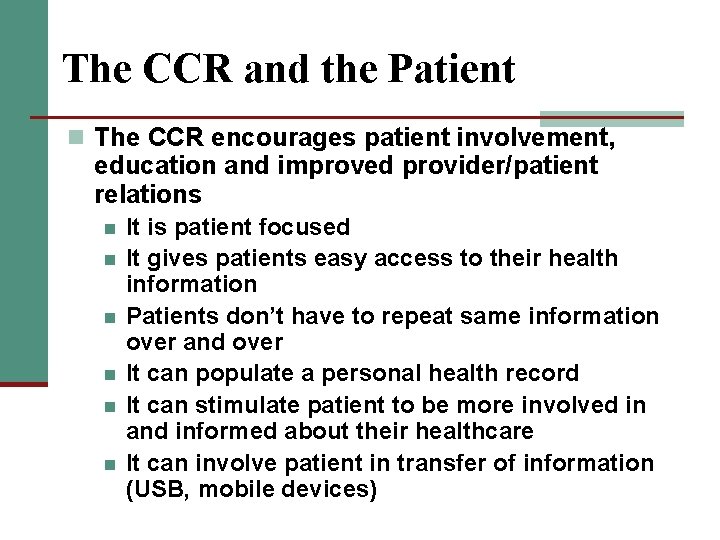
The CCR and the Patient n The CCR encourages patient involvement, education and improved provider/patient relations n n n It is patient focused It gives patients easy access to their health information Patients don’t have to repeat same information over and over It can populate a personal health record It can stimulate patient to be more involved in and informed about their healthcare It can involve patient in transfer of information (USB, mobile devices)

CCR and the Personal Health Record n Widespread interest to use CCR as part of Personal Health Record Government n Payers n Provider institutions n Vendors n Patient advocates n Patients n

m-Health and the CCR n CCR is completed at close of each encounter, so… n Mobile devices and applications offer Point-of-care data entry, access, transmission n Transportability n Connectivity to and interoperability with n n n Source practitioner’s central system Target practitioner Patient’s web-based PHR Secure email communications

Current Status on CCR Development and Adoption n ASTM E 31. 22 Subcommittee on EHR n Preparing CCR for ballot in February 2005 n Only ASTM E 31 and E 31. 28 members may vote n Sponsoring organizations n Promoting CCR adoption among their constituencies and beyond n Vendors Technical Advisory Group n Providing expertise n Participating in demonstration projects n Preparing to adopt standard n ASMT E 31. 20 Subcommittee on Security n Developing CCR security specifications

International Interest n Widespread interest throughout Europe, Asia, Middle East, South America, etc. n ASTM International will explore possibilities with foreign ministries of health, EC, WHO How to adapt/adopt standard n Electronic translation of core data elements n

In Summary n Practitioners, provider institutions, patients, vendors, and other stakeholders perceive the CCR as Relevant n Doable n Transportable and interoperable n Valuable n n They are working together to finalize materials and move toward widespread adoption

How to Become Involved n Join ASTM E 31 Committee on Health Informatics and its E 31. 28 Subcommittee on EHR $75/year n Participate in CCR development n Have voting rights n Free virtual access to CCR standard and all E 31 standards n n Join non-member CCR email list n Notices of meetings and progress

For More Information n Claudia Tessier, CAE, RHIA Co-chair, ASTM E 31. 28 CCR Workgroup 202 -352 -3019 ctessi@attglobal. net THANK YOU!