Overview of the Automated Supplier Change Notification System







- Slides: 9

Overview of the Automated Supplier Change Notification System Last update: 08 -Apr-2015

Supplier Change Control • We recognize that continuous improvement efforts at our suppliers may require changes to manage cost, quality, delivery, and technology • However, implementation of unapproved changes can have unintended effects on our products and therapies, our operations and, more importantly, our customers and patients. • Suppliers must notify Medtronic of changes made to materials, products or processes. 2 | MDT Confidential

Role of the Supplier • Provide adequate advanced notification when possible • Review timeliness of change notification – establish an effective process for triggering notification • Define proactive process to identify changes from your suppliers in a timely manner 3 | MDT Confidential

Previous Medtronic Change Control Process • Previous Medtronic process (mid-2013 and earlier) was burdensome and time consuming • Medtronic is taking steps to standardize and simplify this process where possible • The first stage of this improvement project is to improve the inputs to the process 4 | MDT Confidential

Goal & Scope Project Goal: • Provide a standard input source for change requests − supplierpcn@medtronic. com − Reduce the chance of mishandling • Increase Supplier visibility to the process with automated notifications • Streamline process by eliminating duplicate data entry of change details within Medtronic Project Scope: • Scope is limited to CRHF supply base at this time 5 | MDT Confidential

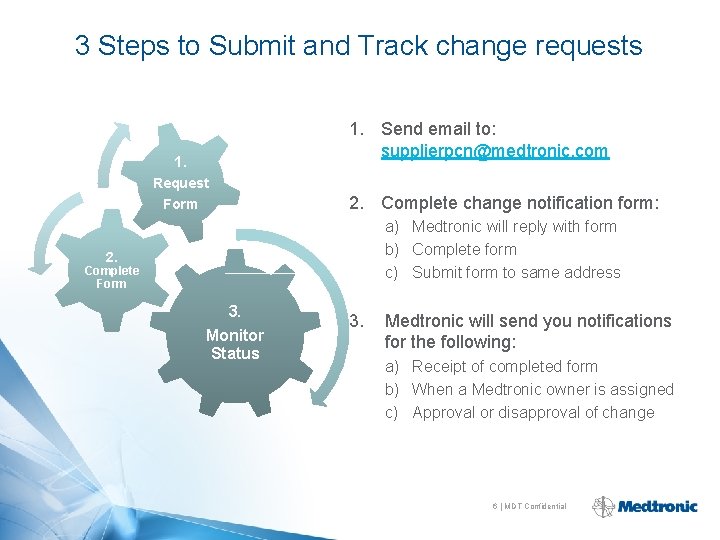
3 Steps to Submit and Track change requests 1. Send email to: supplierpcn@medtronic. com 1. Request Form 2. Complete change notification form: a) Medtronic will reply with form b) Complete form c) Submit form to same address 2. Complete Form 3. Monitor Status 3. Medtronic will send you notifications for the following: a) Receipt of completed form b) When a Medtronic owner is assigned c) Approval or disapproval of change 6 | MDT Confidential

Content Requested For Change • Supplier Details – Who you are and how to contact you • Change Details – What and why the change is being made – What products are impacted • Change Evaluation and Timing – What has been done to evaluate the change and affect on product performance – When the change is needed 7 | MDT Confidential

Benefits to the Supplier • Standardized submission process among CRHF sites • Standard e-mail address for change notification • Visibility and notification of status updates • Traceability to submitted changes 8 | MDT Confidential

Implementation Details • Begin using the supplierpcn@medtronic. com email address and process immediately • If your quality agreement stipulates additional functions be notified of change requests (i. e. buyers), please copy them on the email. • Contact your Medtronic sourcing partner if you have questions 9 | MDT Confidential