Overview of medicines regulation regulatory cooperation and harmonization























- Slides: 25

Overview of medicines regulation: regulatory cooperation and harmonization in the focus Dr Samvel Azatyan Manager, Medicines Regulatory Support Programme Quality Assurance and Safety: Medicines Essential Medicines and Health Products World Health Organization E-mail: azatyans@who. int Advanced Technical Briefing Seminar : Quality Assurance and Safety 1 | of Medicines: Promoting Global Collaboration. 25– 29 June 2012, WHO Headquarters, Geneva

Why regulation of medicines is needed? What is special with medicines, compared to other goods / commodities? § As a rule, patients are not able to make independent judgement about of the QUALITY, SAFETY and EFFICACY; ─Even health professionals have difficulties, unless they are specially trained; Medicines regulation is the totality of all measures - legal, administrative and technical - which governments undertake to ensure the quality, efficacy and safety of medicines, as well as the relevance and accuracy of the product information. Advanced Technical Briefing Seminar : Quality Assurance and Safety 2 | of Medicines: Promoting Global Collaboration. 25– 29 June 2012, WHO Headquarters, Geneva

Government's role is crucial § Governments are obliged to intervene in the activities of the private sector due to public health and safety concerns; § Medical products include: medicines, blood products, vaccines and other biological and biotechnological products, diagnostics, medical devices, traditional medicines and other health-care products; Regulation of medical products in the countries is performed through National Regulatory Authorities (NRAs) Advanced Technical Briefing Seminar : Quality Assurance and Safety 3 | of Medicines: Promoting Global Collaboration. 25– 29 June 2012, WHO Headquarters, Geneva

Role of a National Regulatory Authorities n n The overall objective of a National Regulatory Authorities (NRAs) for medical products is to ensure that all medical products (medicines, vaccines, blood products and other biologicals) and medical devices that are used in a country are of assured quality, safety and efficacy and are accompanied by appropriate information to promote their rational use. NRAs need to be competent, independent, with strong political backing and have clear authority to enforce established regulations. Advanced Technical Briefing Seminar : Quality Assurance and Safety 4 | of Medicines: Promoting Global Collaboration. 25– 29 June 2012, WHO Headquarters, Geneva

The role of a National Regulatory Authority § § § § Medical products regulatory authority means a network (institution) that administers the full spectrum of regulatory activities, including at least the following functions: Marketing authorization for new products and variation of existing authorizations; GMP, GCP, GLP inspections; Licensing and post-license control of manufacturers, wholesalers and other distribution channels; Quality control laboratory testing; Adverse drug reaction monitoring; Provision of drug information and promotion of rational drug use; Enforcement operations; Monitoring of Drug Utilization, etc. Advanced Technical Briefing Seminar : Quality Assurance and Safety 5 | of Medicines: Promoting Global Collaboration. 25– 29 June 2012, WHO Headquarters, Geneva

Main principles of medicines regulation – although, not always observed. . Medicines regulation should: § Have patient in the focus; § Be evidence and science based; § Be risk based; § Bring added value; § Respect interests of stakeholders and real possibilities; § Be transparent but respect confidentiality; § Be effective and flexible; § Be part of broader overall pharmaceutical policy of the country. Advanced Technical Briefing Seminar : Quality Assurance and Safety 6 | of Medicines: Promoting Global Collaboration. 25– 29 June 2012, WHO Headquarters, Geneva

Main principles of medicines regulation – although, not always observed. . …But also keep in mind, that: § Regulations must be attuned not to the wishes, but to available resources (technical, human, financial, etc. ); § Due to the complexity and resource constrains, the requirements developed and successfully implemented in one country may not be equally successful in another country; § … Attempts to apply more sophisticated requirements may have (at least, in short term) serious public health implications. Advanced Technical Briefing Seminar : Quality Assurance and Safety 7 | of Medicines: Promoting Global Collaboration. 25– 29 June 2012, WHO Headquarters, Geneva

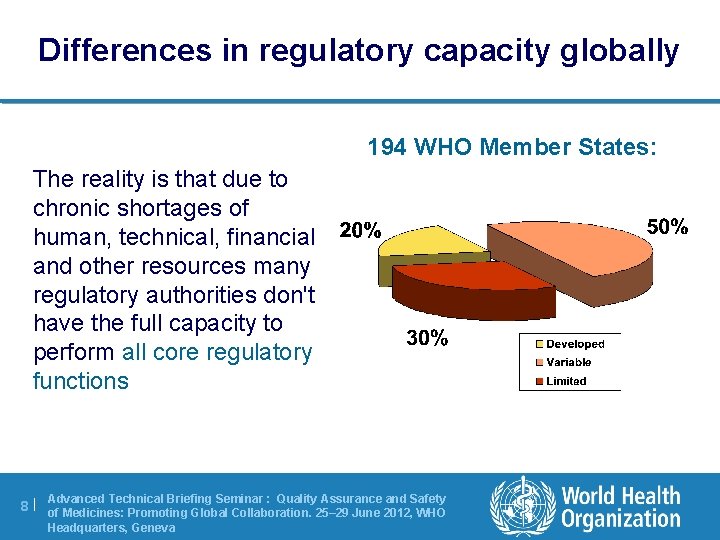
Differences in regulatory capacity globally 194 WHO Member States: The reality is that due to chronic shortages of human, technical, financial and other resources many regulatory authorities don't have the full capacity to perform all core regulatory functions Advanced Technical Briefing Seminar : Quality Assurance and Safety 8 | of Medicines: Promoting Global Collaboration. 25– 29 June 2012, WHO Headquarters, Geneva

Assessments of medicines regulatory systems in 26 sub-Saharan African countries § Guidelines and assessment procedures are not up to international standards and are often of an administrative rather than technical nature; § Wide-ranging exemption clauses exist which are not justified by a risk assessment, for example for public sector imports or donations; § Inadequate resources severely limited technical assessment of dossiers; § In spite of resource constraints only few countries relied on decisions made by other regulators (such as stringent NMRAs or by the WHO Prequalification Programme); § Regulatory decisions by other competent authorities were not widely considered. Advanced Technical Briefing Seminar : Quality Assurance and Safety 9 | of Medicines: Promoting Global Collaboration. 25– 29 June 2012, WHO Headquarters, Geneva

Challenges and problems posed by a lack of appropriate regulation § In many low- and middle income countries most essential medicines are not readily available and accessible due to insufficient regulatory capacity and a lack of harmonized technical requirements for medicines registration § Poor uptake of new and existing health solutions costs millions of lives across low-income countries § A lack of essential medicines contributes to disparities in health and life-expectancy between low- and middle income and high-income countries Advanced Technical Briefing Seminar : Quality Assurance and Safety 10 | of Medicines: Promoting Global Collaboration. 25– 29 June 2012, WHO Headquarters, Geneva

WHO approaches to cope with increasing demands and limited resources § Avoid doing things that do not give added value; § Concentrate on things that do give added value; § Pragmatically and focus on priority issues, which are most relevant for public health (risk-benefit approach); § Increase effectiveness of internal operations; § Co-operate with partners in order to eliminate duplicated activities; § Share your work with others – do what you can do well and let others do what they could do better Advanced Technical Briefing Seminar : Quality Assurance and Safety 11 | of Medicines: Promoting Global Collaboration. 25– 29 June 2012, WHO Headquarters, Geneva

Prioritization of the regulatory activities § Many NMRAs with limited resources may limit their scope of activities to performance of those "core" regulatory functions, which could potentially bring maximum added value to the public health; § Other regulatory functions could be more and more shared with the colleagues in other NMRAs (especially in the frameworks of existing RECs) and relying on the opinions made and decisions taken by other regulators; § This will allow WHO and other partners to prioritize the support efforts and to introduce a system for accreditation (or prequalification) of NMRAs for performance of specific regulatory functions, in accordance with specific international standards. Advanced Technical Briefing Seminar : Quality Assurance and Safety 12 | of Medicines: Promoting Global Collaboration. 25– 29 June 2012, WHO Headquarters, Geneva

More specific "innovative" (or heretic. . ) approach § Introduction of the concept of "levelling" for the pharmaceutical products (essential medicines), according to the "quality-related risk", into: ─Low risk products ─Medium risk products ─High risk products § This will create incentives and motivation for the development, in case if NMRA wishes to "jump" a level up, e. g. , from regulation of low risk products to medium risk products. Advanced Technical Briefing Seminar : Quality Assurance and Safety 13 | of Medicines: Promoting Global Collaboration. 25– 29 June 2012, WHO Headquarters, Geneva

What WHO/MRS is doing to support regulators § Developing evidence - assessments of regulatory systems worldwide (more than 50 NMRAs assessed in all 6 regions); § Providing direct technical support (capacity building, tools and guidance) to regions and countries; § Stimulating / initiating collaboration between regulators from various countries on various regulatory activities; § Promoting and facilitating communication among national/regional regulatory systems using ICDRA, specific network meetings (e. g. WHO Annual Pharmacovigilance Centres meetings, and International Regulatory Cooperation for Herbal Medicines (IRCH); § Promoting regulatory collaboration and harmonization. Advanced Technical Briefing Seminar : Quality Assurance and Safety 14 | of Medicines: Promoting Global Collaboration. 25– 29 June 2012, WHO Headquarters, Geneva

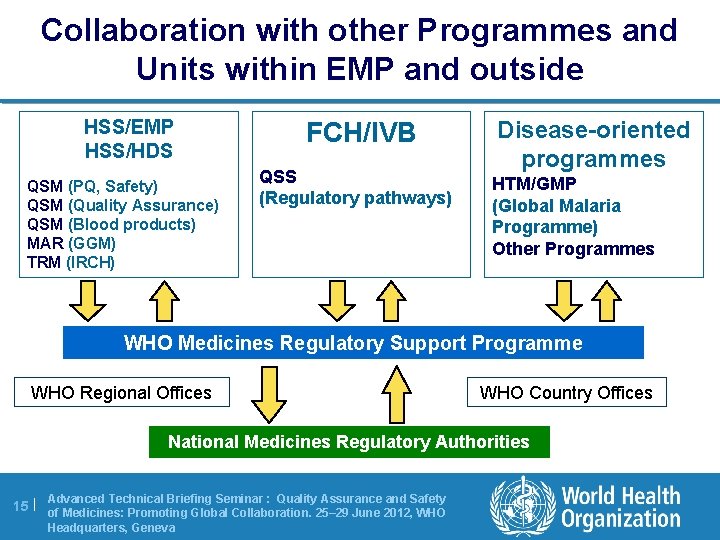
Collaboration with other Programmes and Units within EMP and outside HSS/EMP HSS/HDS QSM (PQ, Safety) QSM (Quality Assurance) QSM (Blood products) MAR (GGM) TRM (IRCH) FCH/IVB QSS (Regulatory pathways) Disease-oriented programmes HTM/GMP (Global Malaria Programme) Other Programmes WHO Medicines Regulatory Support Programme WHO Regional Offices WHO Country Offices National Medicines Regulatory Authorities Advanced Technical Briefing Seminar : Quality Assurance and Safety 15 | of Medicines: Promoting Global Collaboration. 25– 29 June 2012, WHO Headquarters, Geneva

Sharing regulatory information is a key to faster access to medicines § WHO is working with regulators to find out how best to build confidence in regulatory decisions taken by other regulators, including: ─ how to facilitate exchange consolidated information assessments and ─ without challenging their Advanced Technical Briefing Seminar : Quality Assurance and Safety 16 | of Medicines: Promoting Global Collaboration. 25– 29 June 2012, WHO Headquarters, Geneva of about inspections; sovereignty.

Sharing of expertise vs. recognition of decision Acceptance of expertise is not equal to acceptance of decision: § Acceptance of expertise – is sovereign and complex regulatory decision of NRA based on scientific arguments and confidence; – may be applied case to case; – is followed by formal independent decision according to national legislation and mandate of national MRA; § Acceptance of decision – is a formal legal act, frequently requiring international treaties; – may modify liabilities of involved parties and requires legal specification of acceptance and non-acceptance. Advanced Technical Briefing Seminar : Quality Assurance and Safety 17 | of Medicines: Promoting Global Collaboration. 25– 29 June 2012, WHO Headquarters, Geneva

New regulatory approaches Smaller or less resourced regulatory authorities more and more starting to rely on the approvals or opinions issued by the well-resourced regulatory authorities § § § Examples of this type of initiatives include, but are not limited to: WHO PQP - HIV/AIDS, TB, malaria, RH, paediatric therapy; US PEPFAR - HIV/AIDS; EU Article 58 – assessment of products for use outside the European Union territory; Canada's Access to Medicines Regime – assessment of products according to WHO Model List of Essential Medicines; Other – orphan medicines, paediatric medicines. Advanced Technical Briefing Seminar : Quality Assurance and Safety 18 | of Medicines: Promoting Global Collaboration. 25– 29 June 2012, WHO Headquarters, Geneva

What we do mean under harmonization § True harmonization goes further than just development of common documentation; § It requires effective communication and collaboration aimed at building capacity and trust (e. g. , information sharing, recognition and joint working); § In combination, these activities can lead to similar or collaborative approaches to medicines registration; § Can prepare the ground for mutual recognition and/or centralized registration in the longer-term future. Advanced Technical Briefing Seminar : Quality Assurance and Safety 19 | of Medicines: Promoting Global Collaboration. 25– 29 June 2012, WHO Headquarters, Geneva

What we definitely don't mean under harmonization § Harmonization doesn't mean a loss of national sovereignty / autonomy (and certainly not in the early stages); § In all cases the registration decision itself remains firmly in the hands of sovereign nations; § Collaborative mechanisms, such as joint assessments or inspections, does not imply collaborative decision-making! Advanced Technical Briefing Seminar : Quality Assurance and Safety 20 | of Medicines: Promoting Global Collaboration. 25– 29 June 2012, WHO Headquarters, Geneva

Expectations from harmonization § The primary aim of harmonizing technical requirements § § and processes for medicines registration is to improve public health, by increasing timely access to safe and effective medicines of good quality for the treatment of priority diseases. Access will be increased by reducing the time it takes for essential medicines to be registered in‐country, without compromising quality and, potentially, the time taken for essential therapies to reach patients in need. This will require capacity building to ensure transparent, efficient and competent regulatory activities, including assessment of registration dossiers and related inspections, that are able to assure the quality, safety and efficacy of registered medicines. Advanced Technical Briefing Seminar : Quality Assurance and Safety 21 | of Medicines: Promoting Global Collaboration. 25– 29 June 2012, WHO Headquarters, Geneva

Potential public health gains from harmonization § Less risk of being harmed by medicines as gradual improvement of quality, safety and efficacy of products on the markets is expected; § More rapid access to needed medicines - high priority essential medicines, new medicines; § Better value for money - both for out of pocket and public funds, as there will be no waste on substandard and of poor-quality medicines. Advanced Technical Briefing Seminar : Quality Assurance and Safety 22 | of Medicines: Promoting Global Collaboration. 25– 29 June 2012, WHO Headquarters, Geneva

Potential gains have to be balanced with… potential losses § Increased price level of medicines (quality has its price); § Reduced access – not all medicines on the market may meet new harmonized standards; § Increasing price for medicines regulation (harmonization has its price too); § Local industry may not always win; § Wholesale and retail businesses may loose many of the products that were bestsellers for them. Advanced Technical Briefing Seminar : Quality Assurance and Safety 23 | of Medicines: Promoting Global Collaboration. 25– 29 June 2012, WHO Headquarters, Geneva

Conclusions § Medicines regulation is not anymore a "single-player" § § § activity; Prioritization of regulatory activities, work sharing and collaboration can help reduce workload and improve public health by improving overall regulatory performance. It can help to direct the expert knowledge and resources to performance of the functions that can improve public health and facilitate access to essential medicines; Formation of effective networks between regulatory authorities nationally and internationally may facilitate sharing of scarce resources and eliminate duplicating of activities. Advanced Technical Briefing Seminar : Quality Assurance and Safety 24 | of Medicines: Promoting Global Collaboration. 25– 29 June 2012, WHO Headquarters, Geneva

Graphic: www. eauc. org Thank you! www. who. int/medicines Advanced Technical Briefing Seminar : Quality Assurance and Safety 25 | of Medicines: Promoting Global Collaboration. 25– 29 June 2012, WHO Headquarters, Geneva
 Harmonized salary structure
Harmonized salary structure Global harmonisation task force
Global harmonisation task force Data dictionary best practice
Data dictionary best practice Chapter 19 medicines and drugs vocabulary practice answers
Chapter 19 medicines and drugs vocabulary practice answers Summarize roosevelt's approach to environmental problems
Summarize roosevelt's approach to environmental problems Nhs dictionary of medicines and devices
Nhs dictionary of medicines and devices Federal agency for medicines and health products
Federal agency for medicines and health products Veterinary medicines directorate
Veterinary medicines directorate George's marvellous medicine story
George's marvellous medicine story Refrigerant management program
Refrigerant management program The hospital pharmacy generally located at
The hospital pharmacy generally located at 10 herbal plants approved by doh
10 herbal plants approved by doh European directorate for the quality of medicines
European directorate for the quality of medicines Medicines learning portal
Medicines learning portal Ectoparasiticides veterinary medicines
Ectoparasiticides veterinary medicines Nivestime
Nivestime Medicines complete
Medicines complete Cqc medicines management
Cqc medicines management Medicines information centre
Medicines information centre Medicines complete
Medicines complete Pharmac schedule pdf
Pharmac schedule pdf National medicines policy
National medicines policy Ggc medicines
Ggc medicines Agriculture cooperation and farmers welfare
Agriculture cooperation and farmers welfare Chapter 22 regulatory and advisory agencies
Chapter 22 regulatory and advisory agencies Dea number verification
Dea number verification