Overview of Biomaterials By Dr Murtaza Najabat Ali

Overview of Biomaterials By: Dr. Murtaza Najabat Ali (CEng MIMech. E P. E. ) 1

Definitions • Biomaterial - “ is any synthetic material that is used to replace or restore function to a body tissue and is continuously or intermittently in contact with body fluids”. This definition is somewhat restrictive According to a Consensus decision by a panel of experts - “ A Biomaterial is a material to interface with biological system to evaluate, treat, augment, or replace any tissue, organ or function of the body”. • Biomaterials Science - “The physical and biological study of materials and their interaction with the biological environment” 2

FDA Definition: BIOMATERIAL "an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component part, or accessory which is recognized in the official National Formulary, or the United States Pharmacopoeia, or any supplement to them, intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals, or intended to affect the structure or any function of the body of man or other animals, and which does not achieve any of it's primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of any of its primary intended purposes. " 3
FDA Definition: BIOMATERIAL contd. . • It is important to know that the FDA neither approves materials nor maintains a list of approved materials • Although FDA recognizes that many of the currently available biomaterials have vast utility in the fabrication of medical devices, the properties and safety of these materials must be carefully assessed with respect to the specific application in question and its degree of patient contact. • Accurate characterization is an essential step in selecting a material for a medical device, but ultimately the final assessment must be performed on the finished product, under actual use conditions. 4

BIOMATERIALIST OR BIOMATERIALS ENGINEER is responsible to; • develop and characterize the materials used to measure, restore and improve physiologic function, and enhance survival and quality of life. EXAMPLE 1: Are the following items biomaterials ? Why or Why not? (a) Contact Lens (b) Splinter (c) Vascular-Graft (d) Crutches 5
History and Current Status of Biomaterials • Metal dental implants by early civilizations (as early as 200 A. D) • Linen strands used as a suture material ( by the Egyptians) • Unsuccessful earlier surgical procedures • The earliest successful implants were for the skeletal system • Bone plates were introduced by the 1900 s • Adverse effects of earlier Vanadium Steel • S. S and CO-Cr alloys: A success story and the advent of Joint replacement surgeries • PMMA polymers and bone cement appeared in World-war II 6
History and Current Status of Biomaterials contd. . • Today, biomaterials’ market size is over $9 billion per year in USA • Medical devices that possess a large biomaterial component include replacement heart valves, synthetic vascular grafts, hip and knee replacements, heart-lung machines and renal dialysis equipment. • Only in USA, 100, 000 replacement heart valves and 300, 000 vascular grafts implanted every year 7
Biological Response to Biomaterials • One of the major concerns of the Biomaterialist • Some of the reactions from the body, are o Inflammation o Activation of the immune system o Localized blood clotting o Infection o Tumour formation o Implant calcification • Factors such as Type of Material Shape of the implant Bulk Chemical and Mechanical properties Example 2: The biological response to a material is of utmost concern to a biomaterialist. The response must be appropriate for the desired application. For example, calcification of implant materials for bone applications is often sought for proper integration of the implant with the surrounding bone tissue. Would calcification of an artificial heart valve composed of porcine (pig) pericardium be a favourable biological response ? Why or Why not ? 8

Body Conditions § Temperature conditions are not extreme § Chemical and mechanical physiological environment can be extreme, such as o Average non-active person may place 1 to 2. 5 x 106 cycles of stress on his/her hip in one year o For a person 20 to 30 years of age (with a life expectancy of 70 to 80 years), the above cycles of stress is equivalent to approx. 108 cycles of loading in a lifetime § The application of biomaterials for intracorporeal applications, the material must be: o Nontoxic and noncarcinogenic, cause little or no foreign body reaction, and be chemically stable and corrosion resistant o Able to endure large and variable stresses in the highly corrosive environment of the human body o able to be fabricated into intricate shapes and sizes 9
Body Conditions contd. . § Many structural applications of biomaterials in the body require that; o Replacement material fit into a space atleast one-fourth the area of the part being permanently or temporarily replaced or assisted o Implant material may have to withstand loads up to 16 times that which the human bone must withstand o Even in restorative dentistry, high compressive biting forces are combined with large temperature changes and acidity § The chemical structure of the body can cause corrosive attack, which may; o Degrade the implant o Release of ions and particles 10
Requirements of Biomaterials A biomaterial must be: • inert or specifically interactive • biocompatible • mechanically and chemically stable or • biodegradable (for specific applications) • processable (for manufacturability) • nonthrombogenic (if blood‐contacting) • sterilizable 11
Different Biomaterials Attributes Bioactive material is biocompatible but plays a more aggressive role in the body – Recruits specific interactions between the material and the surrounding tissue – Encourage tissue integration Bioinert material is biocompatible and does not elicit a significant biological response Biocompatibility refers to any construct that can be brought into direct contact without: – Causing a systemic toxic reaction – Have tumorigenic response 12
Different Biomaterials Attributes The meaning of the words Biodegradable and Bioresorbable are often used misleadingly in the literature • Biodegradable Materials o Refers to the breakdown of the material by biological elements and gives degradation by-products or fragments • Bioresorbable Materials o refers to the degradation of the material by natural pathways and which reflects total elimination of the foreign material (implant) or degradation by-products with no residual side effects 13
Biocompatibility According to consensus definition “Biocompatibility is the ability of a material to perform with an appropriate host response in a specific application” • It involves the acceptance of an artificial implant by the surrounding tissues and by the body as a whole • Biocompatible materials do not; o Provoke an abnormal inflammatory response o Irritate the surrounding structures o Incite allergic or immunologic reactions o Do not cause cancer 14
Biocompatibility Example 3: Is the “ideal” biomaterial always one which is chemically inert? Why? Example 4: Would the “ideal” hip joint implant be as strong as possible ? Why? Example 5: Give atleast five potential “undesirable” biomaterial reactions 15

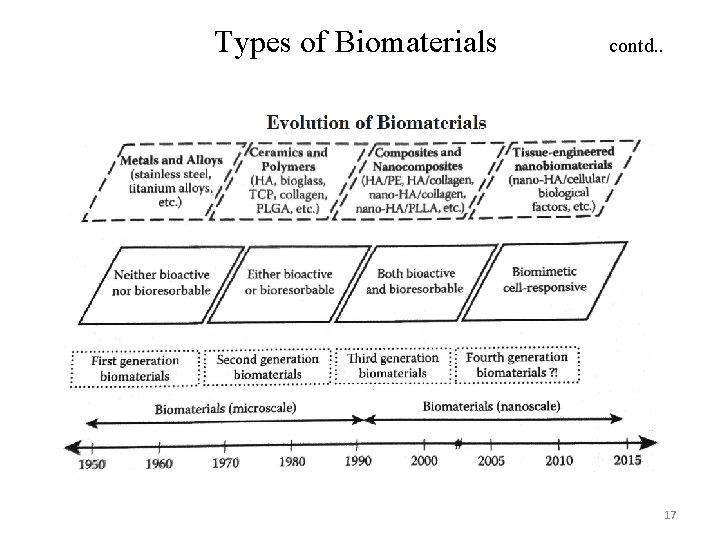
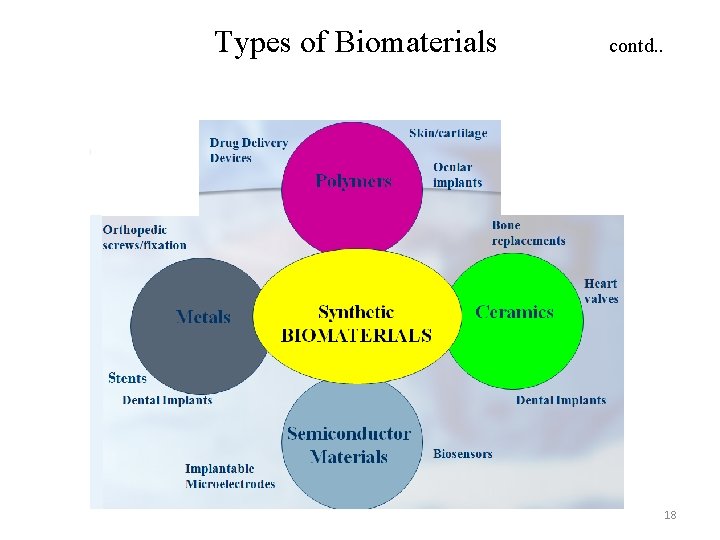
Types of Biomaterials can be divided into four major classes of materials Biomaterials Polymers Metals Ceramics Natural Composites Fifth Class of Biomaterials 16
Types of Biomaterials contd. . 17
Types of Biomaterials contd. . 18
Types of Biomaterials contd. . • Polymers o Consists of repeating units strung together in long chains o Represent the largest class of biomaterials o Natural o Synthetic o Hydrophobic o Hydrophilic o Water swelling materials (Hydrogels) o Biostable o Biodegradable 19
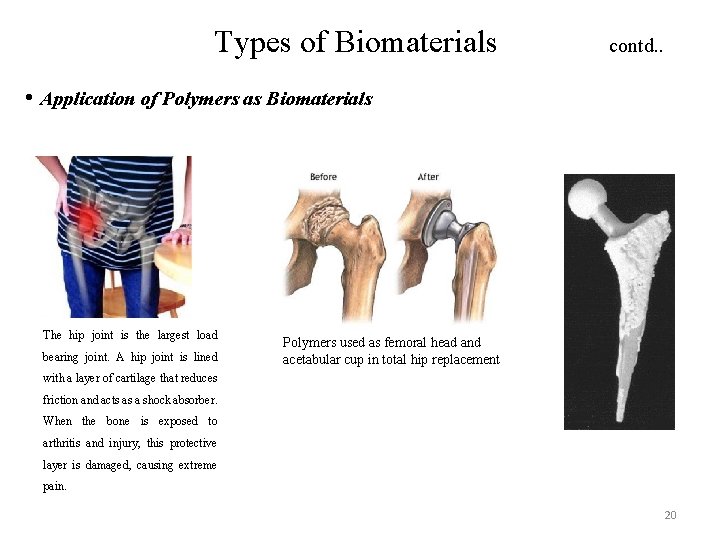
Types of Biomaterials contd. . • Application of Polymers as Biomaterials The hip joint is the largest load bearing joint. A hip joint is lined Polymers used as femoral head and acetabular cup in total hip replacement with a layer of cartilage that reduces friction and acts as a shock absorber. When the bone is exposed to arthritis and injury, this protective layer is damaged, causing extreme pain. 20
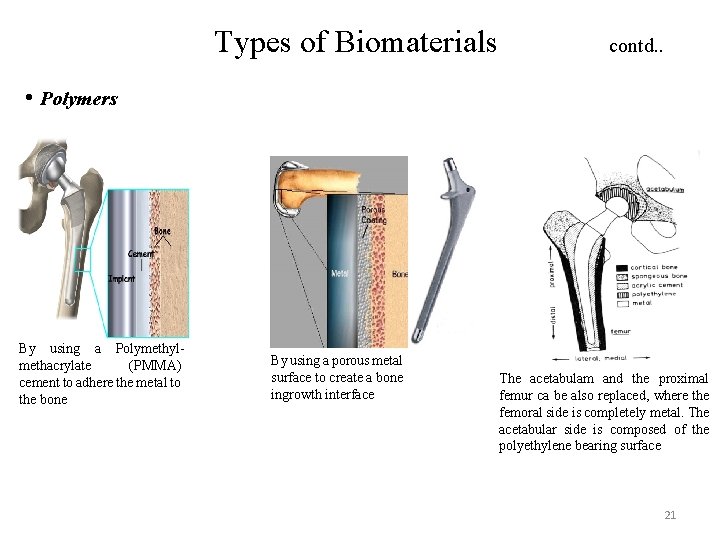
Types of Biomaterials contd. . • Polymers By using a Polymethylmethacrylate (PMMA) cement to adhere the metal to the bone By using a porous metal surface to create a bone ingrowth interface The acetabulam and the proximal femur ca be also replaced, where the femoral side is completely metal. The acetabular side is composed of the polyethylene bearing surface 21
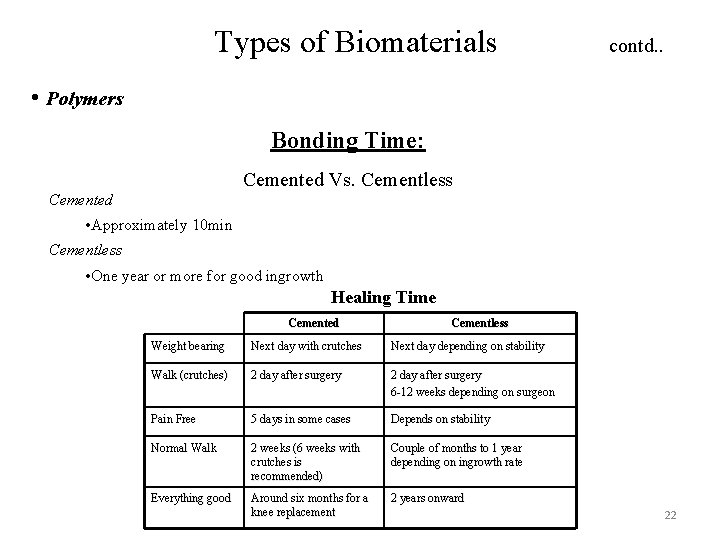
Types of Biomaterials contd. . • Polymers Bonding Time: Cemented Vs. Cementless Cemented • Approximately 10 min Cementless • One year or more for good ingrowth Healing Time Cemented Cementless Weight bearing Next day with crutches Next day depending on stability Walk (crutches) 2 day after surgery 6 -12 weeks depending on surgeon Pain Free 5 days in some cases Depends on stability Normal Walk 2 weeks (6 weeks with crutches is recommended) Couple of months to 1 year depending on ingrowth rate Everything good Around six months for a knee replacement 2 years onward 22
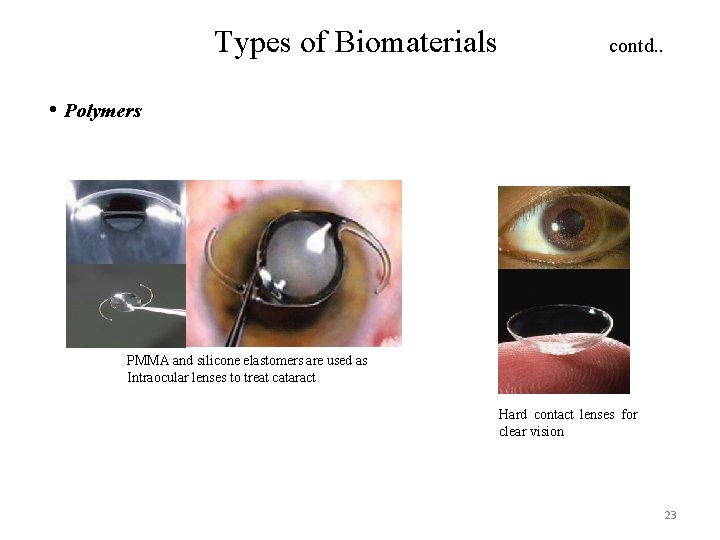
Types of Biomaterials contd. . • Polymers PMMA and silicone elastomers are used as Intraocular lenses to treat cataract Hard contact lenses for clear vision 23
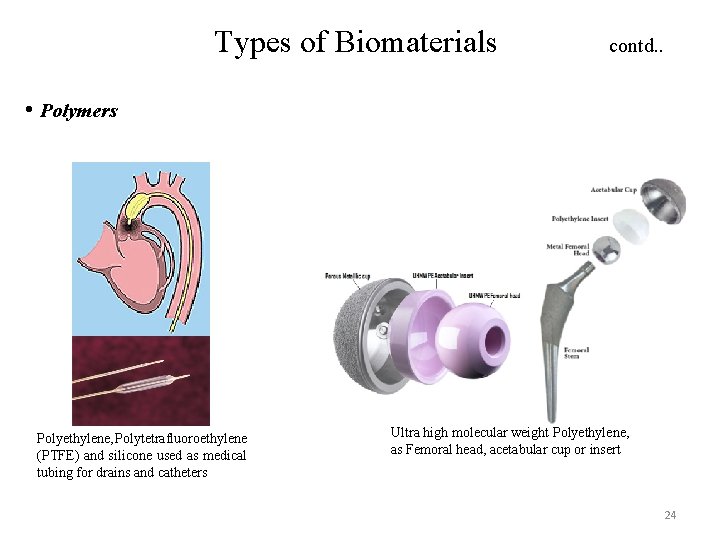
Types of Biomaterials contd. . • Polymers Polyethylene, Polytetrafluoroethylene (PTFE) and silicone used as medical tubing for drains and catheters Ultra high molecular weight Polyethylene, as Femoral head, acetabular cup or insert 24
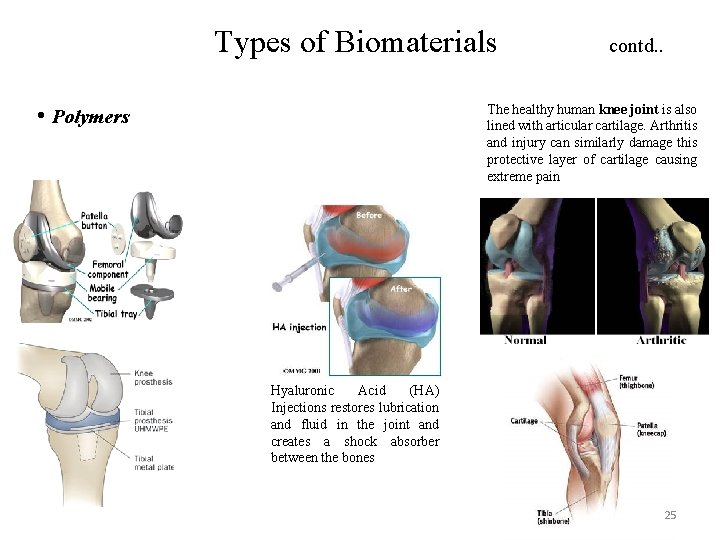
Types of Biomaterials • Polymers contd. . The healthy human knee joint is also lined with articular cartilage. Arthritis and injury can similarly damage this protective layer of cartilage causing extreme pain Hyaluronic Acid (HA) Injections restores lubrication and fluid in the joint and creates a shock absorber between the bones 25
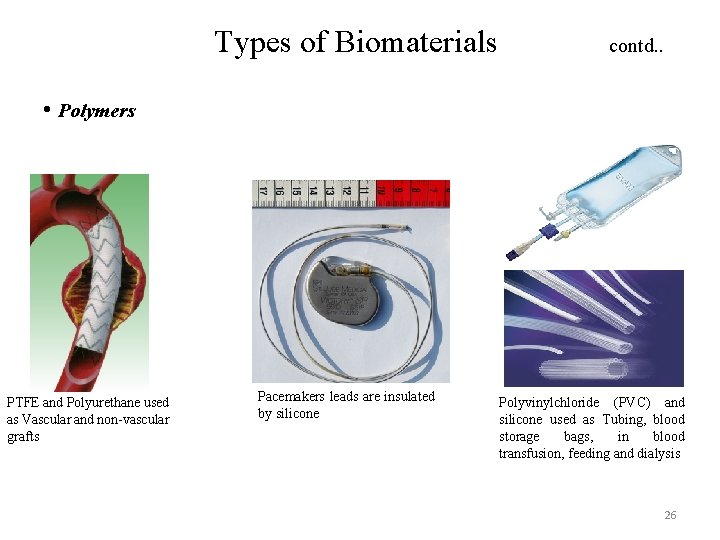
Types of Biomaterials contd. . • Polymers PTFE and Polyurethane used as Vascular and non-vascular grafts Pacemakers leads are insulated by silicone Polyvinylchloride (PVC) and silicone used as Tubing, blood storage bags, in blood transfusion, feeding and dialysis 26
Types of Biomaterials contd. . • Metals • Load bearing implants and internal fixation devices; • When processed suitably contribute high tensile, high fatigue and high yield strengths; • Properties depend on the processing method and purity of the metal. • When metallic biomaterials were first used in biomedical applications, the only requirement was to achieve a suitable combination of physical properties to match those of the replaced tissue with a minimal toxic response of the host • Few concepts were gradually introduced with time as requirements for metallic biomaterials in the design of implantable devices, such as ü Foreign body reaction (particularly due to wear debris) ü Stress shielding ü Biocompatibility ü Bioactivity, and ü Osteoinduction 27
Types of Biomaterials contd. . • Metals Although many metals and alloys are used for medical device applications, the most commonly employed are • Stainless steels • Cobalt-base alloys • Commercially pure Titanium and Titanium alloys • Nickel- Titanium alloys, and some • Noble metals (Au, Pt, Pd) 28
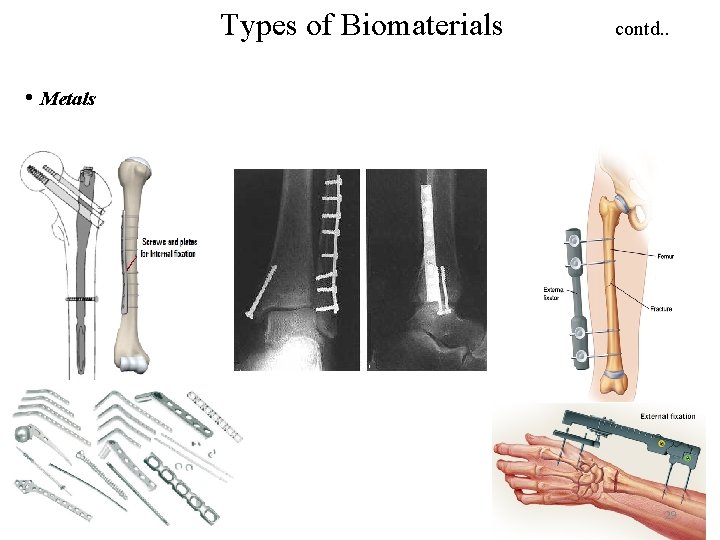
Types of Biomaterials contd. . • Metals 29
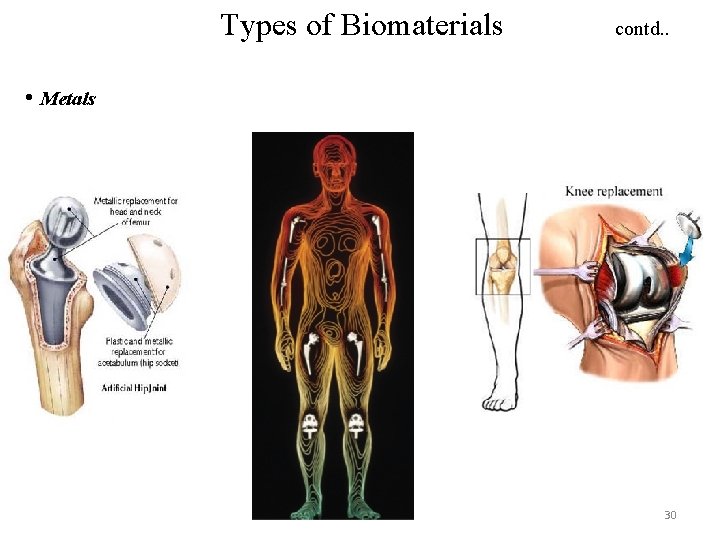
Types of Biomaterials contd. . • Metals 30
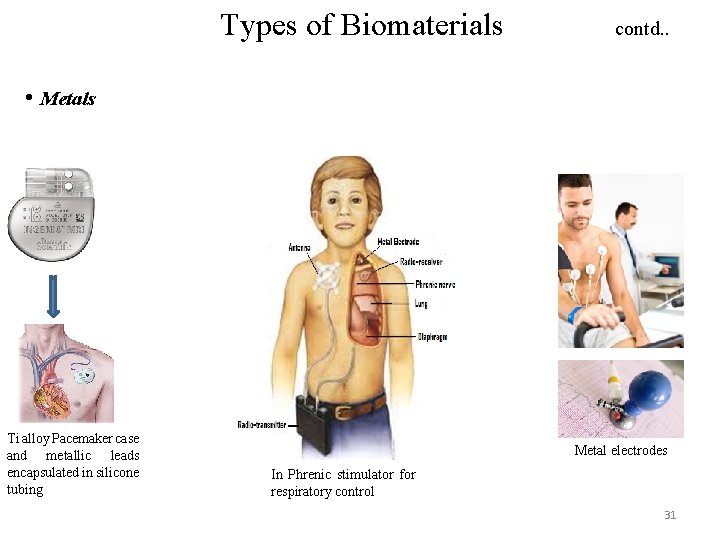
Types of Biomaterials contd. . • Metals Ti alloy Pacemaker case and metallic leads encapsulated in silicone tubing Metal electrodes In Phrenic stimulator for respiratory control 31
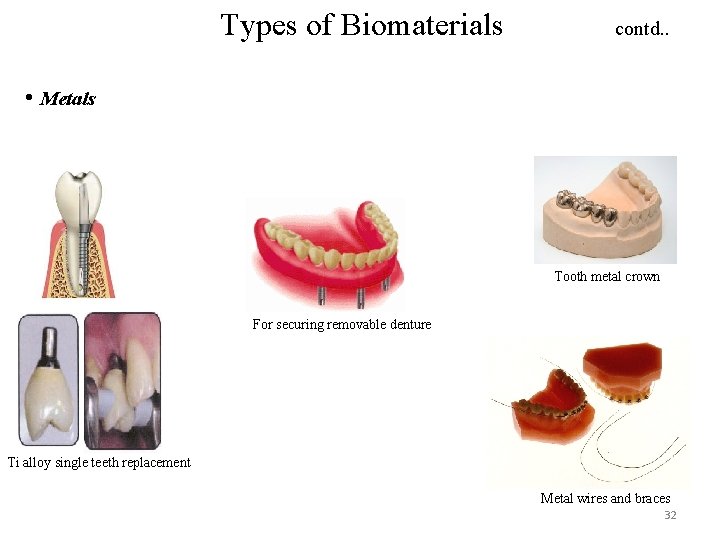
Types of Biomaterials contd. . • Metals Tooth metal crown For securing removable denture Ti alloy single teeth replacement Metal wires and braces 32
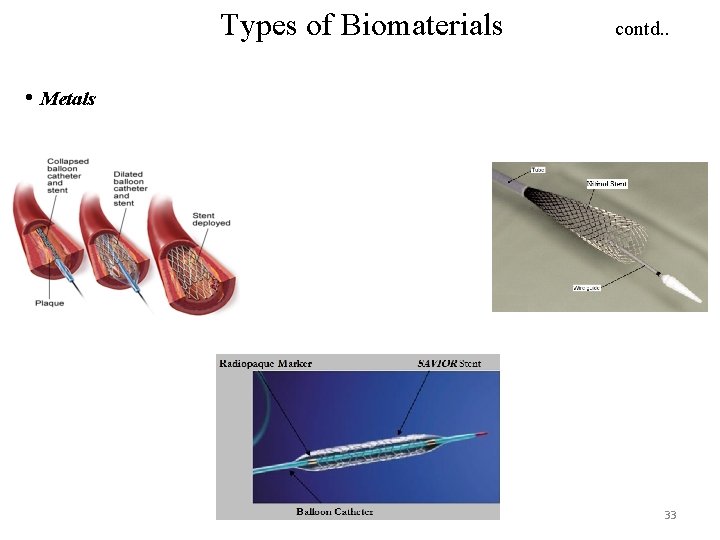
Types of Biomaterials contd. . • Metals 33
Types of Biomaterials contd. . • Ceramics • Bone bonds well to them • Exhibit minimum foreign body reaction • High stiffness • Low friction and wear coefficients But • Low fracture toughness Owing to its Brittle nature • Low impact resistance 34
Types of Biomaterials contd. . • Ceramics • Alumina (Al 2 O 3) • Zirconia • Several porous ceramics (Ca. CO 3) The most common ceramic materials can be classified (both as cements and ceramics) as ; Calcium Bioactive Glasses Glass Ceramics Phosphates (Ca. Ps) (BGs) • Hydroxyapatite Ca 10(PO 4)6(OH)2 • A range of suitable Ca. Ps (amorphous Ca. P (ACP), dicalcium phosphate (DCP), tricalcium phosphate (α-TCP, β-TCP), tetracalcium phosphate (TTCP) e. t. c. ) • Several BGs formulations containing e. g. Si. O 2 as network former and Na 2 O, Ca. O and P 2 O 5 as modifying oxides (Bioglass 45 S 5) 35
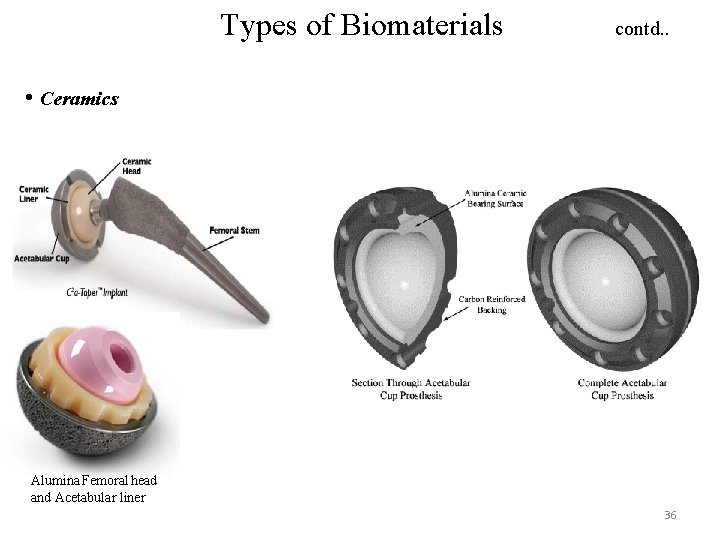
Types of Biomaterials contd. . • Ceramics Alumina Femoral head and Acetabular liner 36
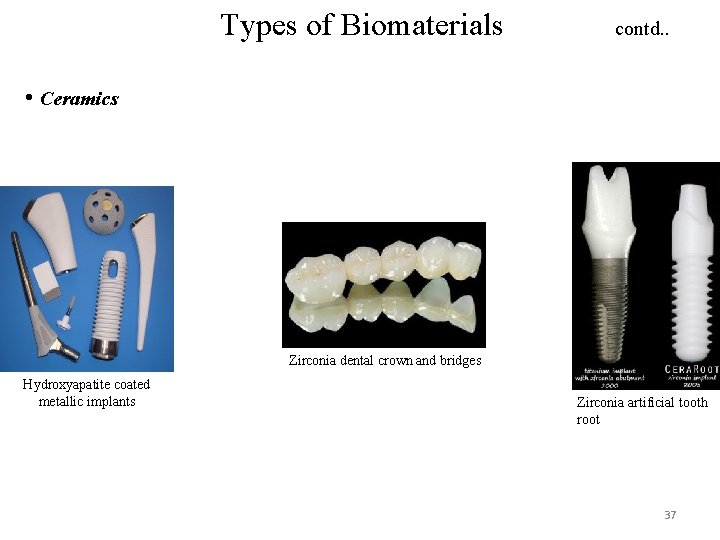
Types of Biomaterials contd. . • Ceramics Zirconia dental crown and bridges Hydroxyapatite coated metallic implants Zirconia artificial tooth root 37
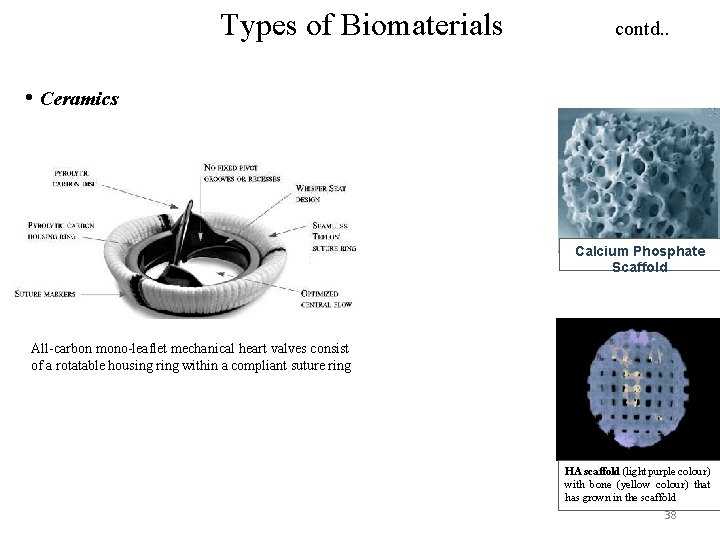
Types of Biomaterials contd. . • Ceramics Calcium Phosphate Scaffold All-carbon mono-leaflet mechanical heart valves consist of a rotatable housing ring within a compliant suture ring HA scaffold (light purple colour) with bone (yellow colour) that has grown in the scaffold 38
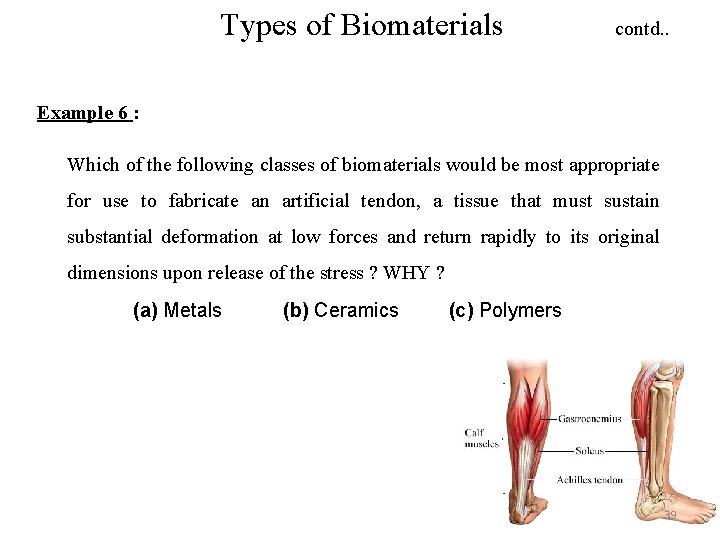
Types of Biomaterials contd. . Example 6 : Which of the following classes of biomaterials would be most appropriate for use to fabricate an artificial tendon, a tissue that must sustain substantial deformation at low forces and return rapidly to its original dimensions upon release of the stress ? WHY ? (a) Metals (b) Ceramics (c) Polymers 39
- Slides: 39