Overview of atomic structure and dimension one popular




- Slides: 11

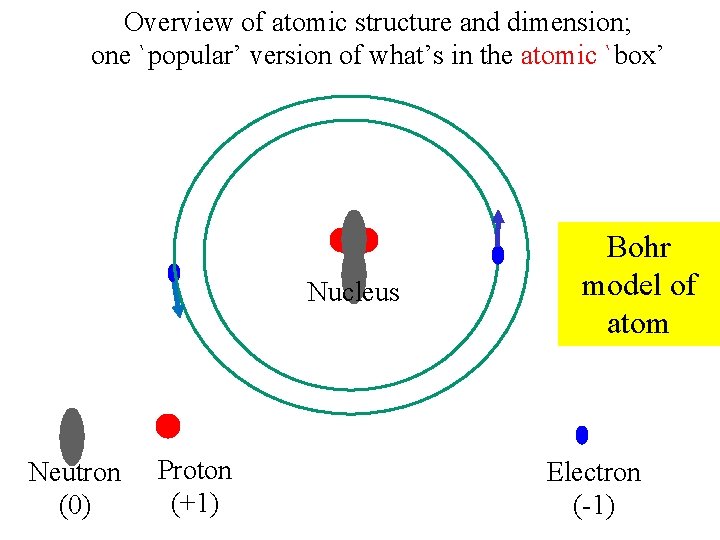
Overview of atomic structure and dimension; one `popular’ version of what’s in the atomic `box’ Nucleus Neutron (0) Proton (+1) Bohr model of atom Electron (-1)

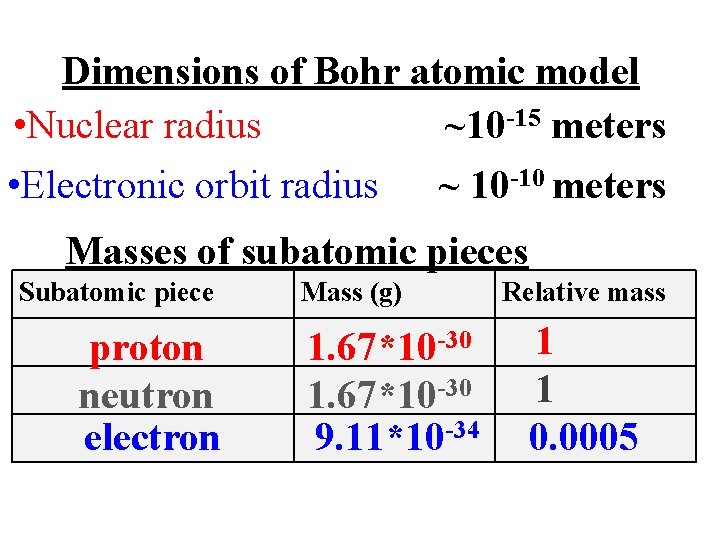
Dimensions of Bohr atomic model • Nuclear radius ~10 -15 meters • Electronic orbit radius ~ 10 -10 meters Masses of subatomic pieces Subatomic piece proton neutron electron Mass (g) 1. 67*10 -30 9. 11*10 -34 Relative mass 1 1 0. 0005

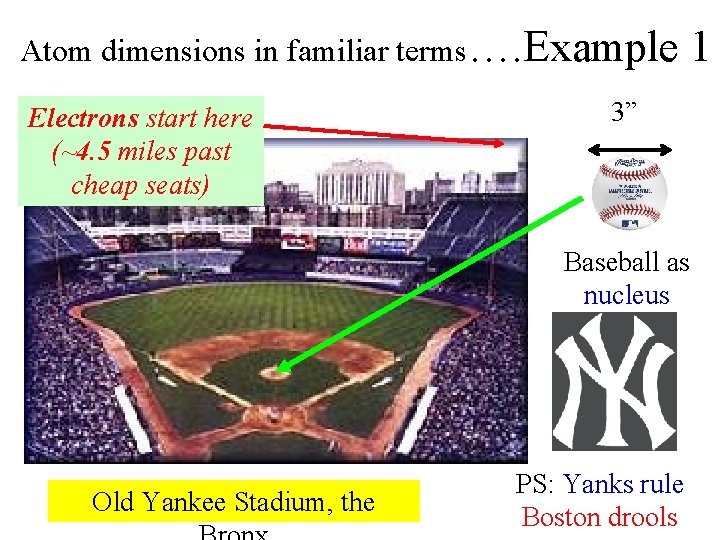
Atom dimensions in familiar terms…. Example Electrons start here (~4. 5 miles past cheap seats) 1 3” Baseball as nucleus Old Yankee Stadium, the PS: Yanks rule Boston drools

Atom dimensions in familiar terms… Example 2 Nucleus=Target store in Omaha Atomic nucleus=Super. Where Target store first here Omaha, Nebraska (~75 electrons meter dia. ) appear

OTHER WAYS TO `GRASP’ ATOMIC DIMENSIONS • paper + scissors • Soda can + string *don’t shower ERICA* JUSTIN*

Popular picture of the Bohr atom reviewed 10 -10 m 10 -15 m Nucleus Electron radius = Nuclear radius 10 -10 = 10 -15 10+5 = 100, 000 Neutron (0) Proton (+1) Electron (-1)

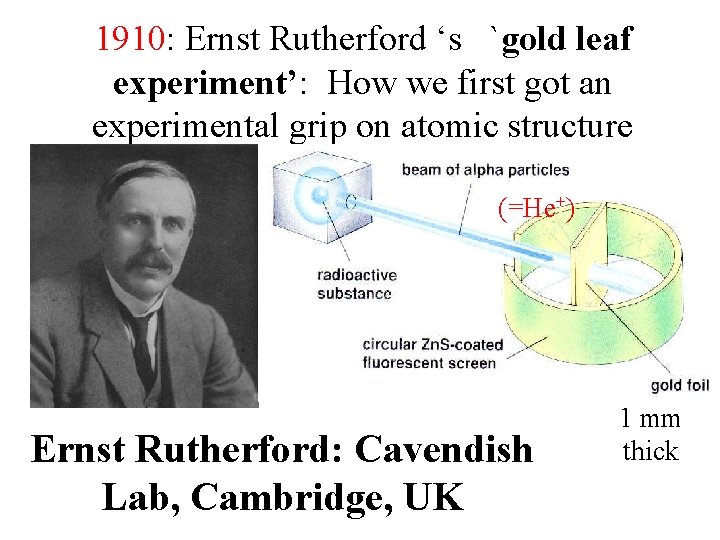
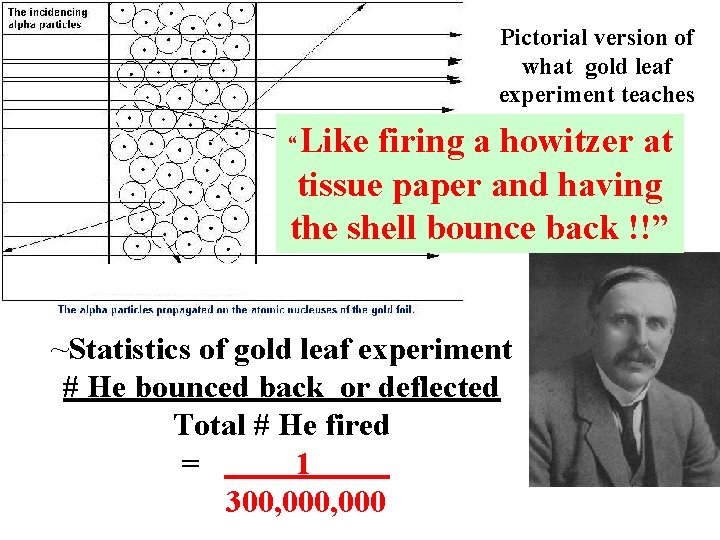
1910: Ernst Rutherford ‘s `gold leaf experiment’: How we first got an experimental grip on atomic structure (=He+) Ernst Rutherford: Cavendish Lab, Cambridge, UK 1 mm thick

Pictorial version of what gold leaf experiment teaches “Like firing a howitzer at tissue paper and having the shell bounce back !!” ~Statistics of gold leaf experiment # He bounced back or deflected Total # He fired = 1 300, 000

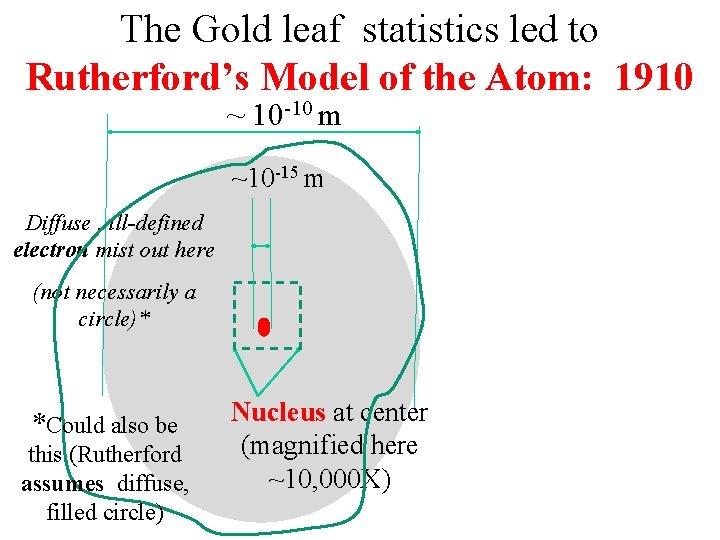
The Gold leaf statistics led to Rutherford’s Model of the Atom: 1910 ~ 10 -10 m ~10 -15 m Diffuse , ill-defined electron mist out here (not necessarily a circle)* *Could also be this (Rutherford assumes diffuse, filled circle) Nucleus at center (magnified here ~10, 000 X)

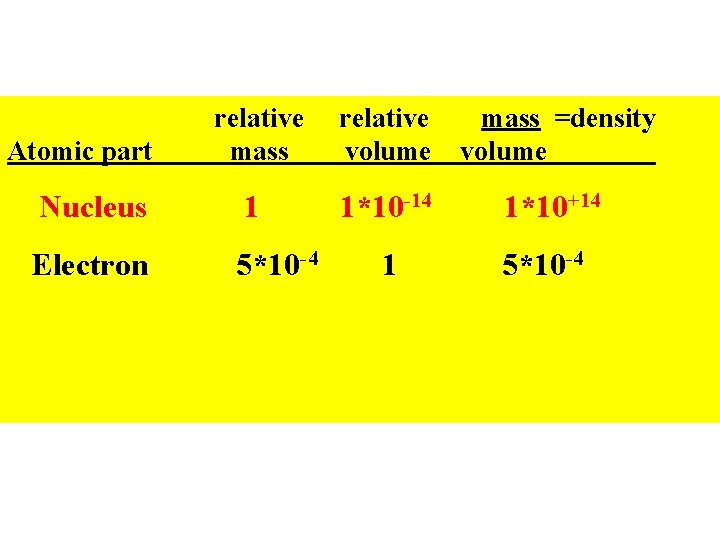
relative mass relative volume mass =density volume Nucleus 1 1*10 -14 1*10+14 Electron 5*10 -4 1 5*10 -4 Atomic part

WHERE WE’VE BEEN TODAY IN CHEM 1114 LECTURE 2 WEDNESDAY 29 AUGUST 2012 • BOHR’S IMAGE & DIMENSIONS • DOWN-TO-EARTH MODELINGS OF THE ATOM • MATH CHECK: SCIENTIFIC NOTATION REVIEW • HOW WE KNOW THE DIMENSIONS: GOLD LEAF EXPERIMENT • PRE-TEST