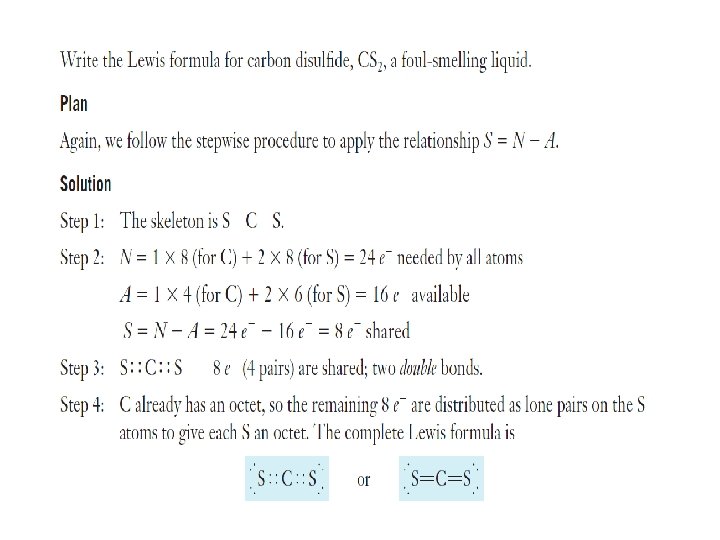
Overlapping orbitals Draw orbital diagrams for F F

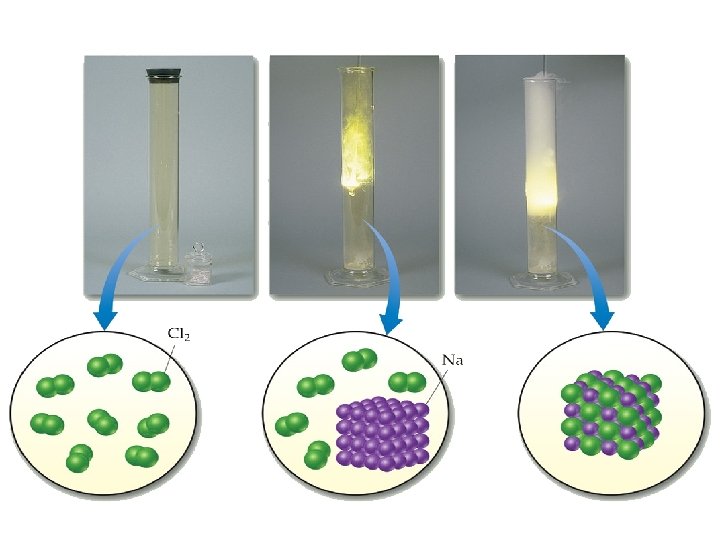

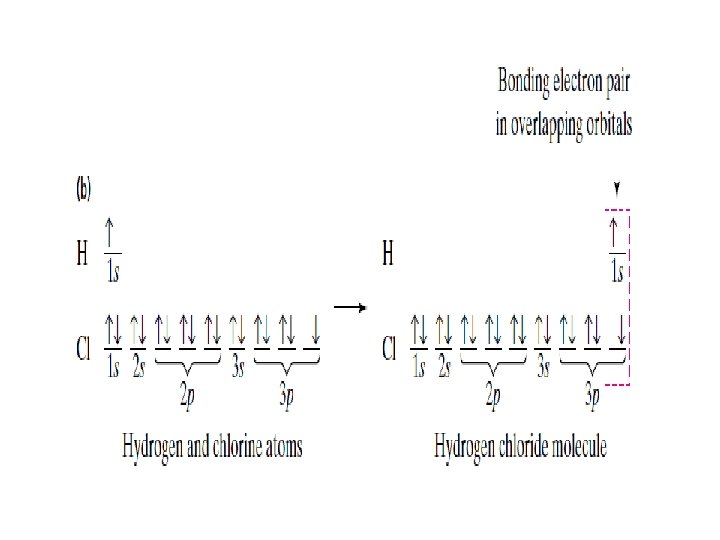
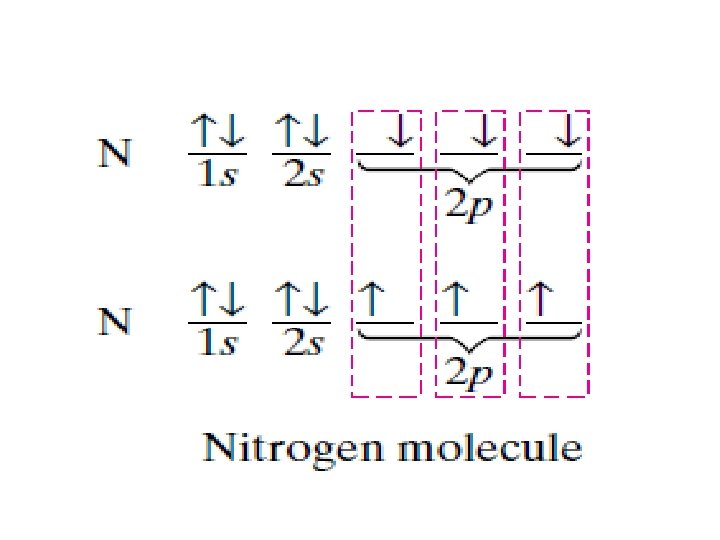

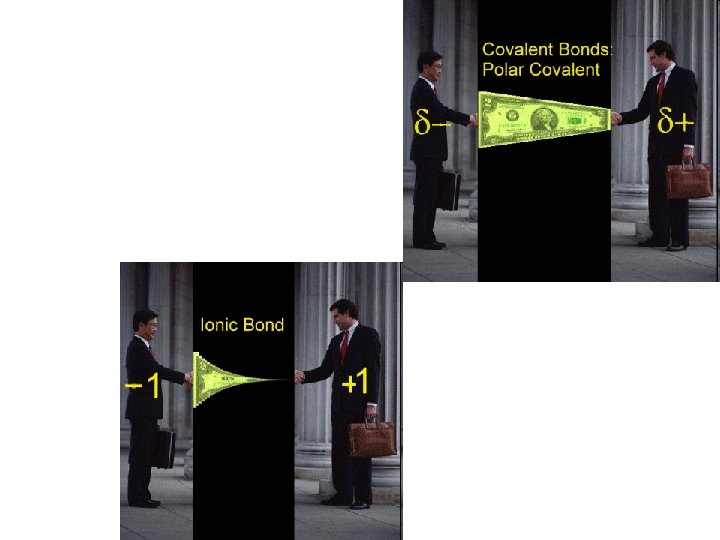
Overlapping orbitals Draw orbital diagrams for F + F, H + O, Li + F • 1 s 2 s 2 p 2 p F 2 2 s 1 s 1 s 1 s 2 s H 2 O 2 p 1 s 1 s 2 s 2 p 2 s 1 s Li. F is ionic (metal + non-metal)
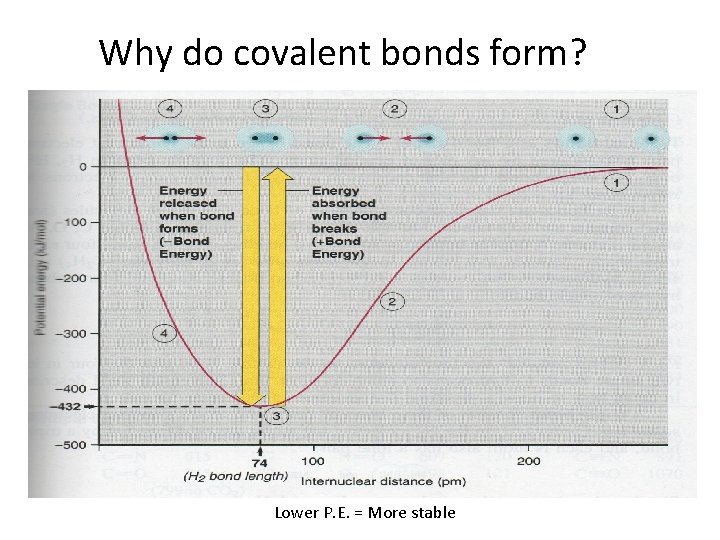
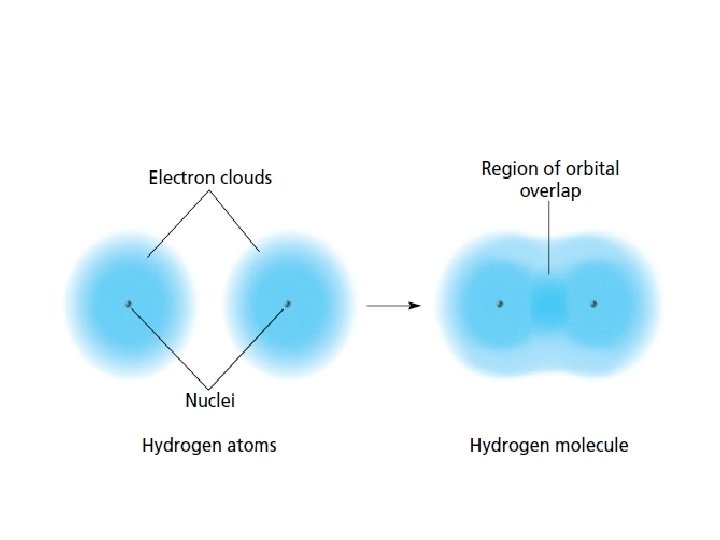
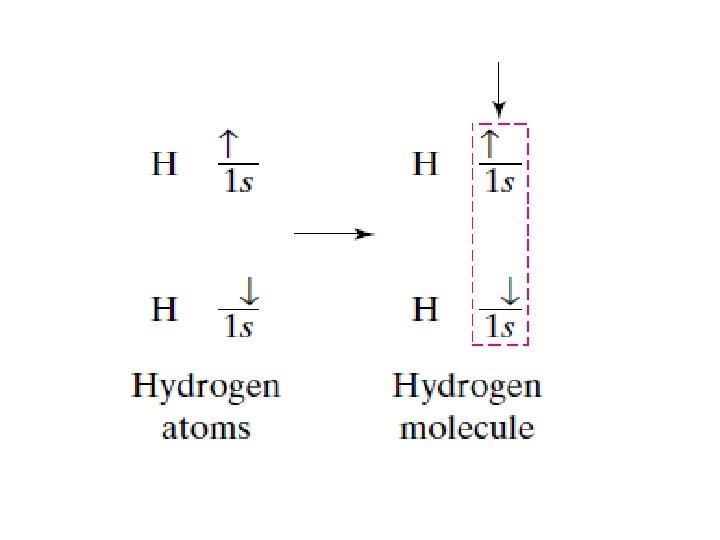
Why do covalent bonds form? Lower P. E. = More stable
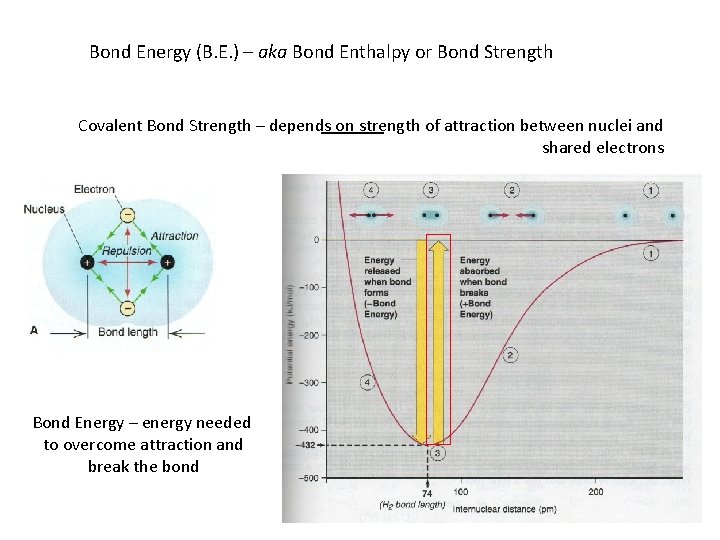
Bond Energy (B. E. ) – aka Bond Enthalpy or Bond Strength Covalent Bond Strength – depends on strength of attraction between nuclei and shared electrons Bond Energy – energy needed to overcome attraction and break the bond
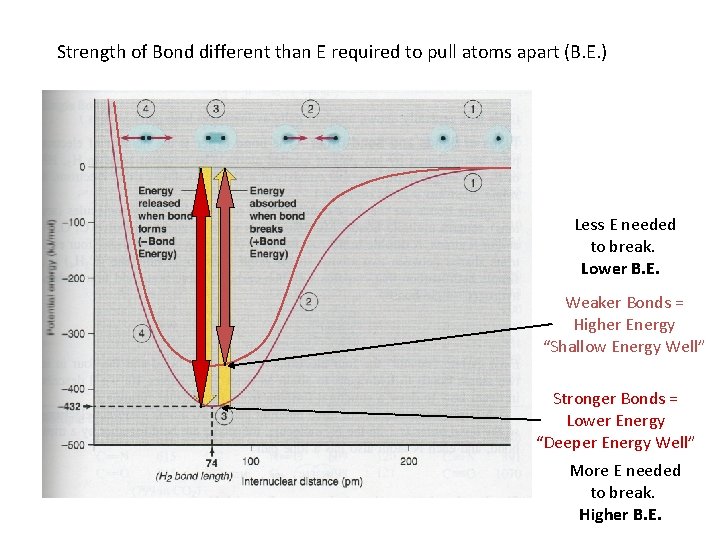
Strength of Bond different than E required to pull atoms apart (B. E. ) Less E needed to break. Lower B. E. Weaker Bonds = Higher Energy “Shallow Energy Well” Stronger Bonds = Lower Energy “Deeper Energy Well” More E needed to break. Higher B. E.

Section 9. 3: Covalent Bonding Bond Type (Single, Double, Triple) also matters Same two elements, different B. E. Nuclei more attracted to 2 shared pairs of e- than one shared pair of e-. Higher bond order = Shorter bond length = Higher Bond Energy

Section 9. 3: Covalent Bonding Periodic Table Trends Without Detailed Bond Lengths The closer the atoms, the stronger the bond. Bond Energy: C—F > C—Cl > C—Br




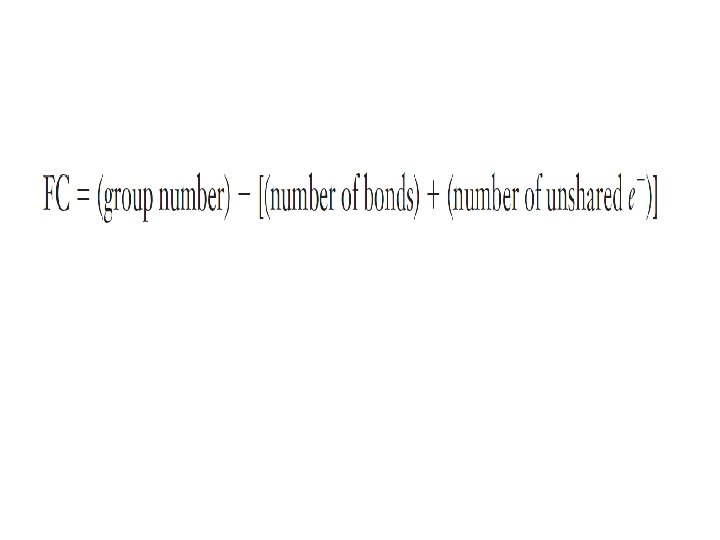

ﺑﺮﺍی ﻭﺭﻭﺩ ﺑﻪ ﺷﺒکﻪ آﻤﻮﺯﺷی ﺩﺍﻧﺸﺠﻮیﺎﻥ Title کﻠیک کﻨیﺪ Lorem ipsum dolor sit amet, • consectetuer adipiscing elit. Vivamus et magna. Fusce sed sem sed magna suscipit egestas. Lorem ipsum dolor sit amet, • 808090901000 consectetuer adipiscing elit. Vivamus et magna. Fusce sed sem ﻟﻄﻔﺎ آﺪﺭﺱ ﻣﺎ ﺭﺍ ﺑﻪ ﺧﺎﻃﺮ ﺩﺍﺷﺘﻪ ﺑﺎﺷیﺪ sed magna suscipit egestas. MADSG. COM
- Slides: 25