Outline How is ferromagnetism manifested What are the











- Slides: 31

Outline • How is ferromagnetism manifested? • What are the types of magnetism? • What is Fe 3 O 4 – spinel? • What is nanoscience? • How do we make ferrofluids?

We will have a Monday class next week • • Turn in extra credit Writing exercises will be returned Possible chance for regaining lost points SRTI evaluations

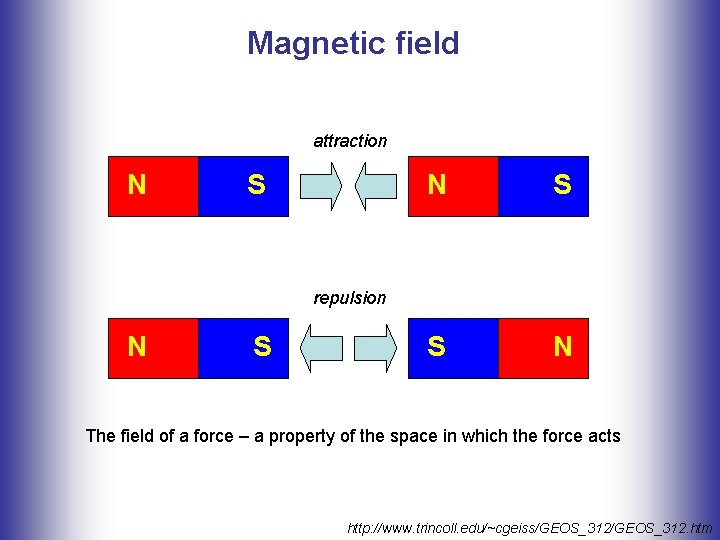
Magnetic field attraction N S S N repulsion N S The field of a force – a property of the space in which the force acts http: //www. trincoll. edu/~cgeiss/GEOS_312. htm

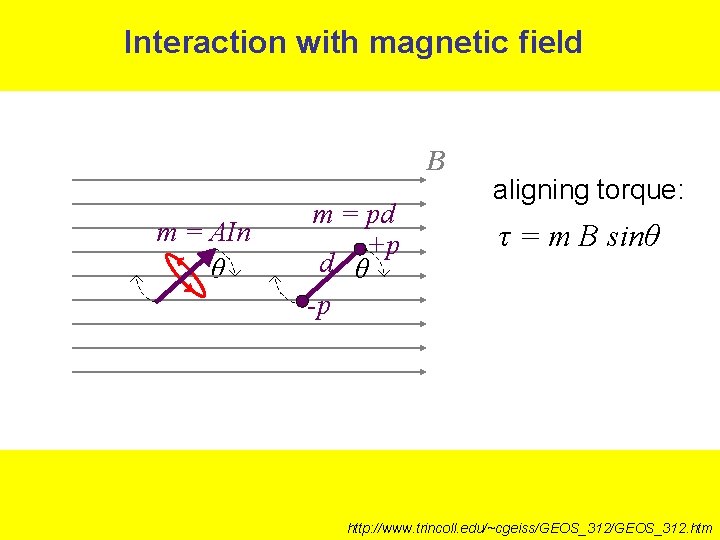
Interaction with magnetic field B m = AIn θ m = pd +p d θ -p aligning torque: τ = m B sinθ http: //www. trincoll. edu/~cgeiss/GEOS_312. htm

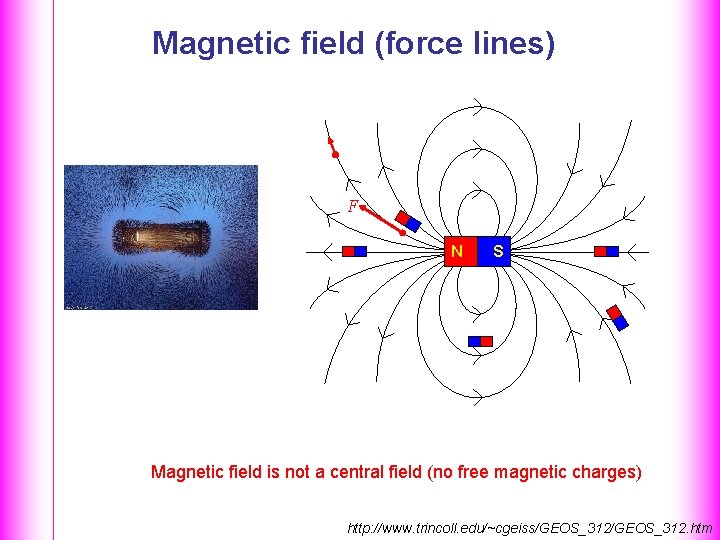
Magnetic field (force lines) F N S Magnetic field is not a central field (no free magnetic charges) http: //www. trincoll. edu/~cgeiss/GEOS_312. htm

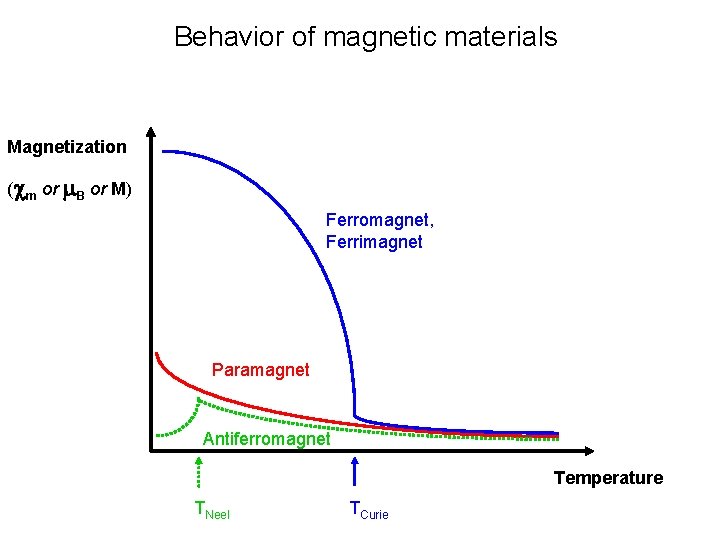
Behavior of magnetic materials Magnetization (cm or m. B or M) Ferromagnet, Ferrimagnet Paramagnet Antiferromagnet Temperature TNeel TCurie

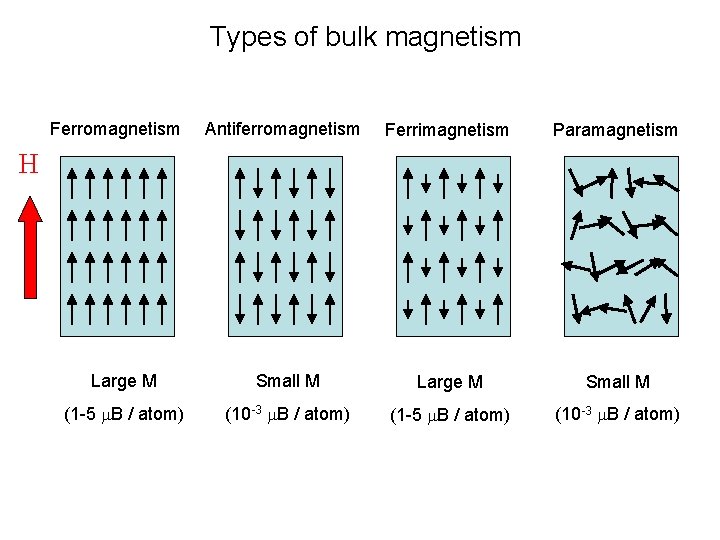
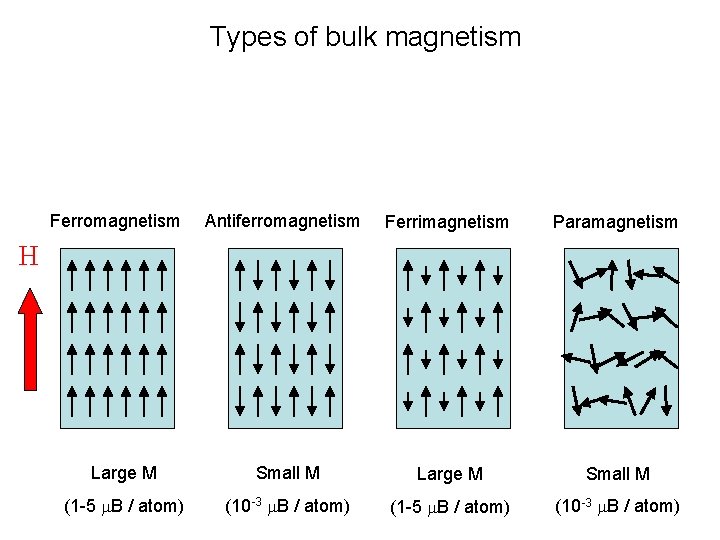
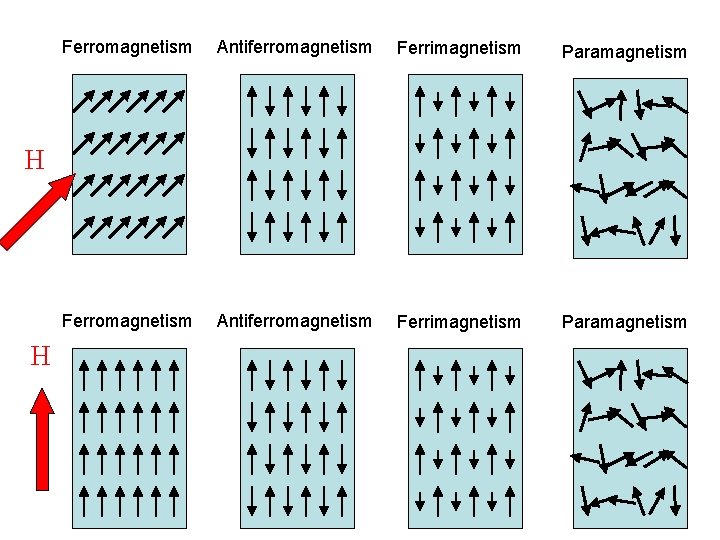
Types of bulk magnetism Ferromagnetism Antiferromagnetism Ferrimagnetism Paramagnetism Large M Small M (1 -5 m. B / atom) (10 -3 m. B / atom) H

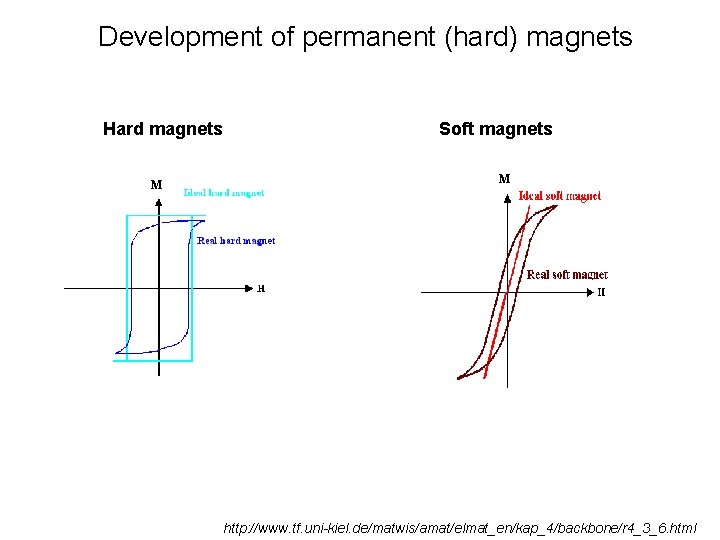
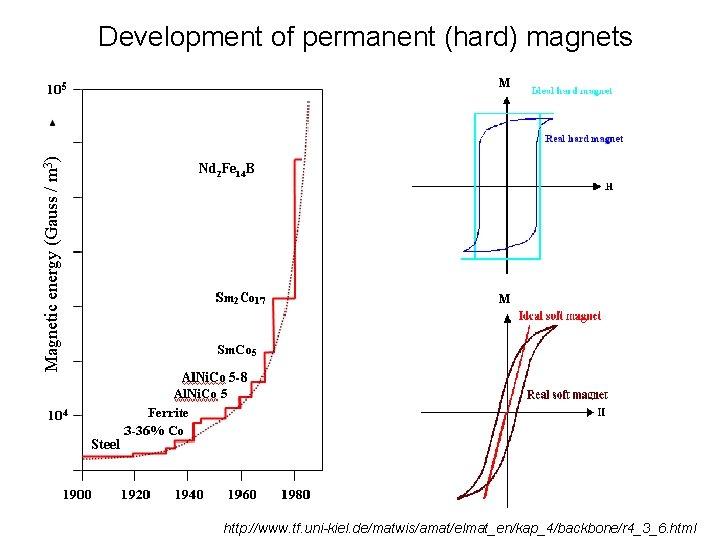
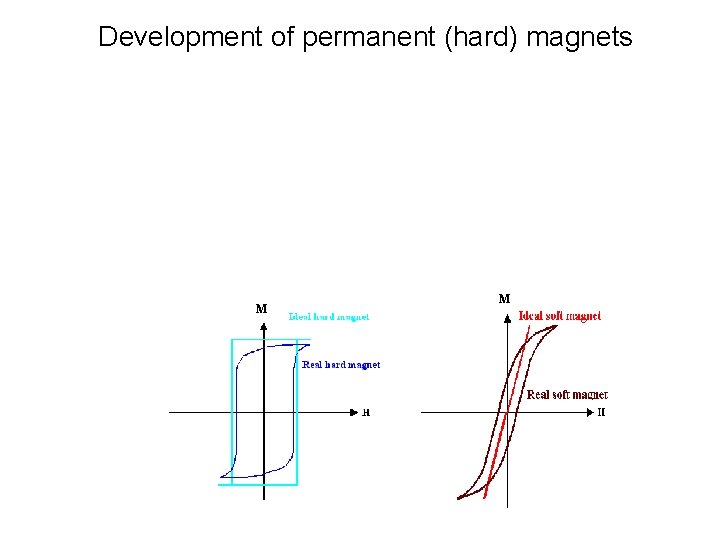
Development of permanent (hard) magnets Hard magnets M Soft magnets M http: //www. tf. uni-kiel. de/matwis/amat/elmat_en/kap_4/backbone/r 4_3_6. html

What is nanoscience?

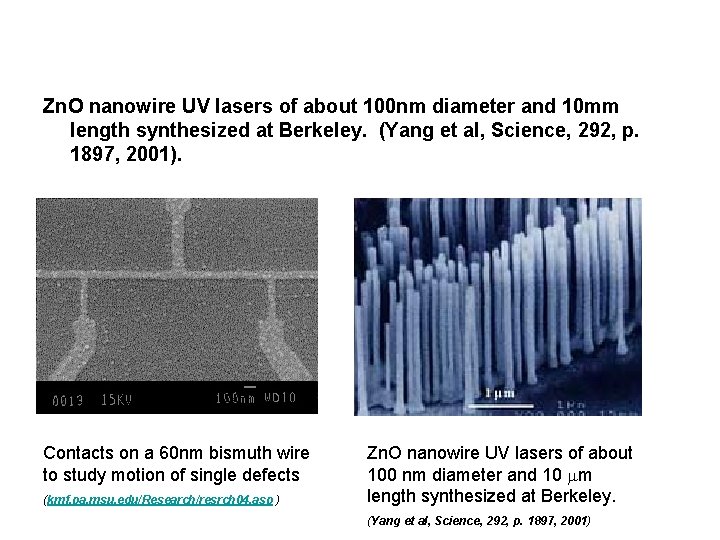
Zn. O nanowire UV lasers of about 100 nm diameter and 10 mm length synthesized at Berkeley. (Yang et al, Science, 292, p. 1897, 2001). Contacts on a 60 nm bismuth wire to study motion of single defects (kmf. pa. msu. edu/Research/resrch 04. asp ) Zn. O nanowire UV lasers of about 100 nm diameter and 10 mm length synthesized at Berkeley. (Yang et al, Science, 292, p. 1897, 2001)

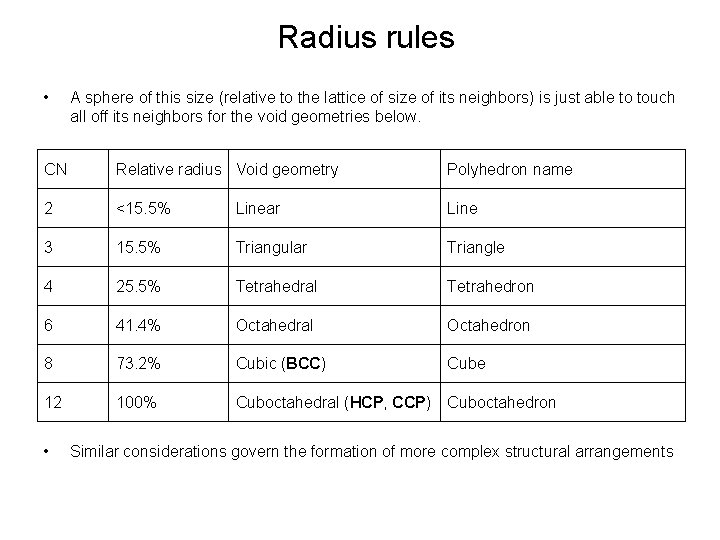
Radius rules • A sphere of this size (relative to the lattice of size of its neighbors) is just able to touch all off its neighbors for the void geometries below. CN Relative radius Void geometry Polyhedron name 2 <15. 5% Linear Line 3 15. 5% Triangular Triangle 4 25. 5% Tetrahedral Tetrahedron 6 41. 4% Octahedral Octahedron 8 73. 2% Cubic (BCC) Cube 12 100% Cuboctahedral (HCP, CCP) Cuboctahedron • Similar considerations govern the formation of more complex structural arrangements

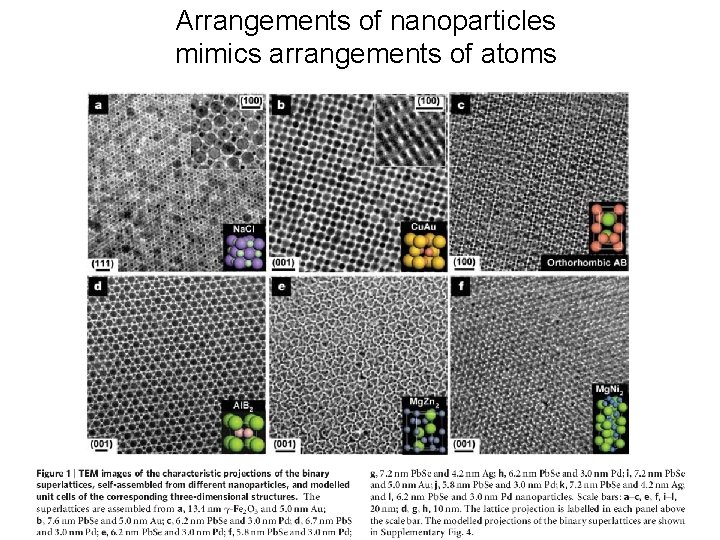
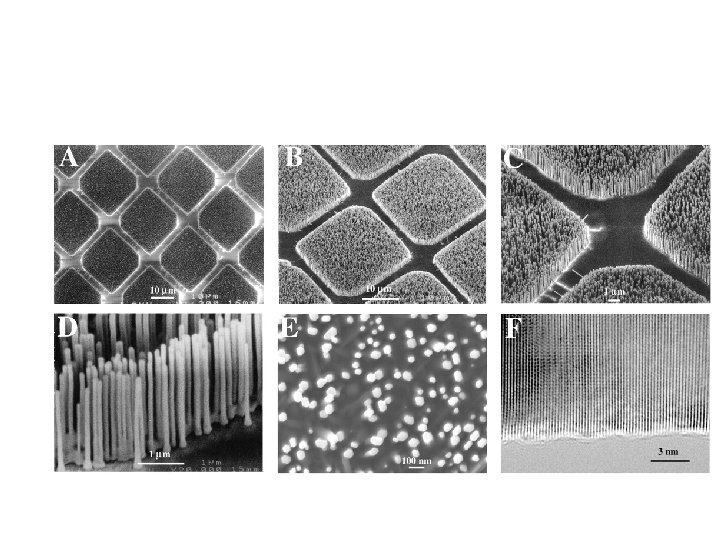
Arrangements of nanoparticles mimics arrangements of atoms

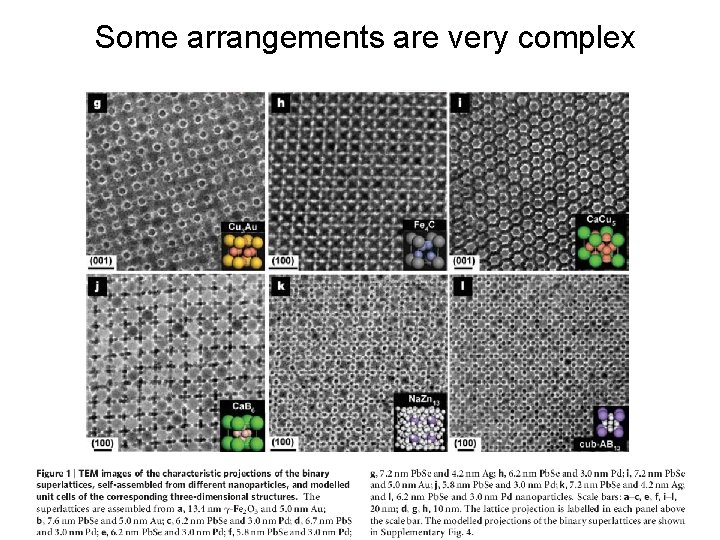
Some arrangements are very complex

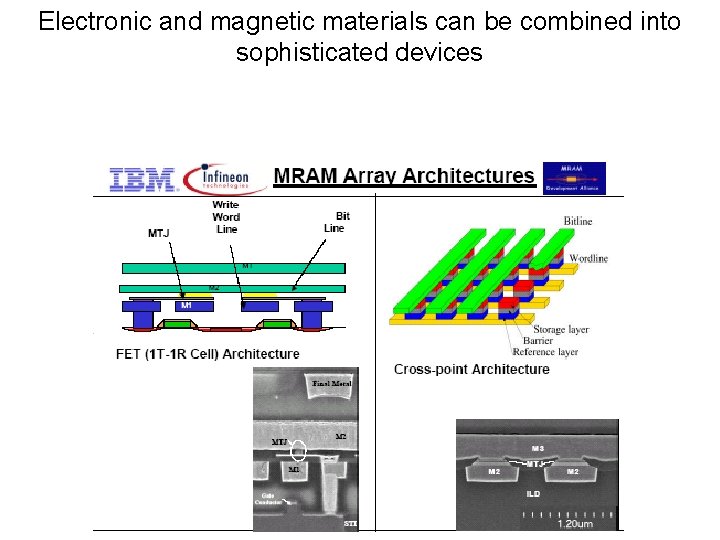
Electronic and magnetic materials can be combined into sophisticated devices

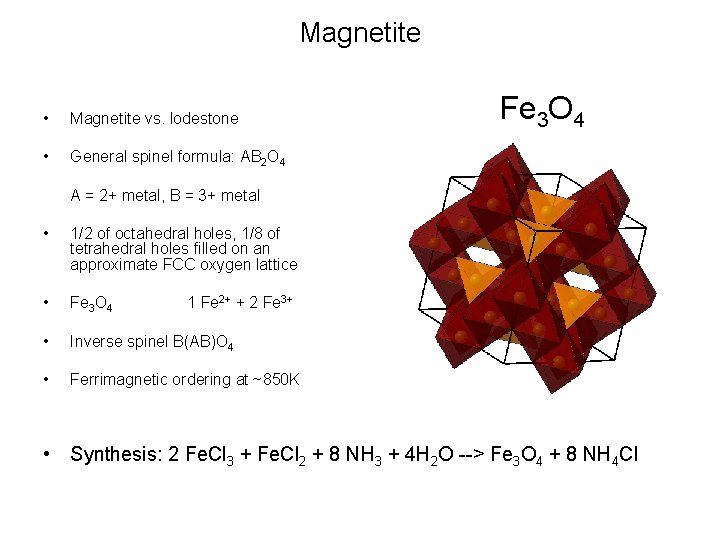
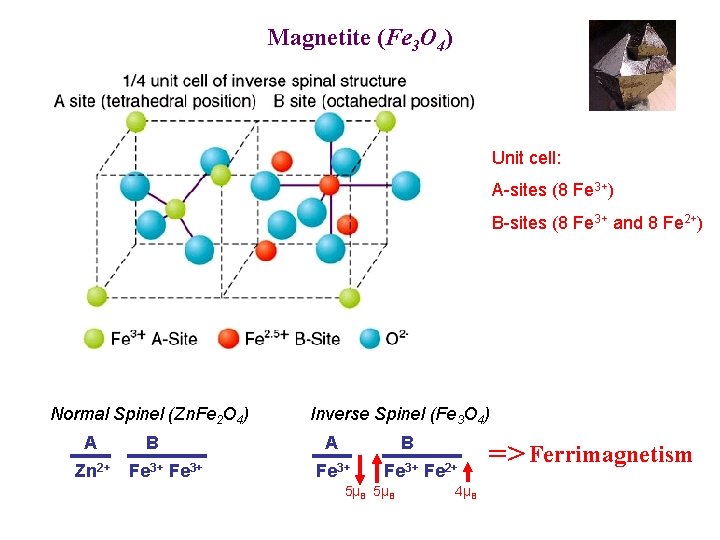
Magnetite • Magnetite vs. lodestone • General spinel formula: AB 2 O 4 Fe 3 O 4 A = 2+ metal, B = 3+ metal • 1/2 of octahedral holes, 1/8 of tetrahedral holes filled on an approximate FCC oxygen lattice • Fe 3 O 4 • Inverse spinel B(AB)O 4 • Ferrimagnetic ordering at ~850 K 1 Fe 2+ + 2 Fe 3+ • Synthesis: 2 Fe. Cl 3 + Fe. Cl 2 + 8 NH 3 + 4 H 2 O --> Fe 3 O 4 + 8 NH 4 Cl

Magnetite (Fe 3 O 4) Unit cell: A-sites (8 Fe 3+) B-sites (8 Fe 3+ and 8 Fe 2+) Normal Spinel (Zn. Fe 2 O 4) A Zn 2+ B Fe 3+ Inverse Spinel (Fe 3 O 4) A Fe 3+ B Fe 3+ 5µB Fe 2+ 4µB => Ferrimagnetism

Development of permanent (hard) magnets Magnetic energy (Gauss / m 3) M Nd 2 Fe 14 B M Steel http: //www. tf. uni-kiel. de/matwis/amat/elmat_en/kap_4/backbone/r 4_3_6. html

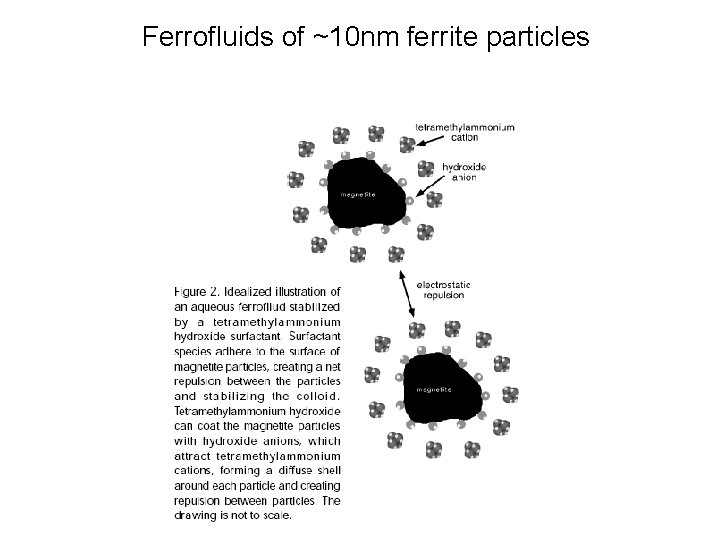
Ferrofluids of ~10 nm ferrite particles

Ferrofluids



Types of bulk magnetism Ferromagnetism Antiferromagnetism Ferrimagnetism Paramagnetism Large M Small M (1 -5 m. B / atom) (10 -3 m. B / atom) H

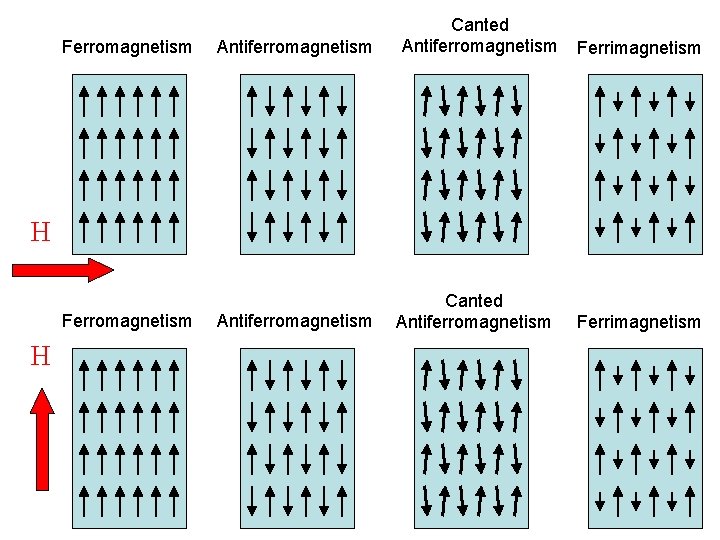
Ferromagnetism Antiferromagnetism Canted Antiferromagnetism Ferrimagnetism H Ferromagnetism H Antiferromagnetism Canted Antiferromagnetism Ferrimagnetism



Ferromagnetism Antiferromagnetism Ferrimagnetism Paramagnetism H H

Types of magnetism Ferromagnetism Antiferromagnetism Ferrimagnetism Paramagnetism Large M Small M (1 -5 m. B / atom) (10 -3 m. B / atom) H


Ferrofluid topics • Magnetic dipoles, not monopoles like charges • Field gradient - emphasized by magnetic field lines • A “test dipole” will aligns itself parallel to magnetic field lines

Development of permanent (hard) magnets M M
