Outline Biosafety Biological hazard Good microbiological practice GMP


Outline • • Biosafety Biological hazard Good microbiological practice (GMP) Safety equipment Personal protective equipment (PPE) and clothing Bio-spill kit Biosafety cabinet Microbial Classification: risk and biohazard group

Biosafety The discipline addressing the safe handling and containment of infectious microorganisms and hazardous biological materials Laboratory worker, stakeholder and environment protection Ed. HHS Publication No. (CDC) 21 -1112 (2009) Ref: Suda Louirirtchanakul, Faculty of Medicine Sirirraj hospital, Mahidol University
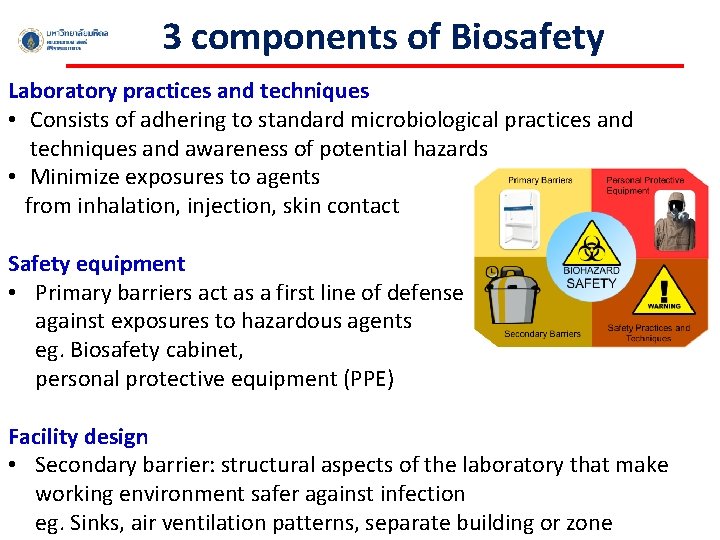
3 components of Biosafety Laboratory practices and techniques • Consists of adhering to standard microbiological practices and techniques and awareness of potential hazards • Minimize exposures to agents from inhalation, injection, skin contact Safety equipment • Primary barriers act as a first line of defense against exposures to hazardous agents eg. Biosafety cabinet, personal protective equipment (PPE) Facility design • Secondary barrier: structural aspects of the laboratory that make working environment safer against infection eg. Sinks, air ventilation patterns, separate building or zone

Biological hazard: Diagnosis laboratory § Hazard related with the pathogen § Hazard related with human/animal cells
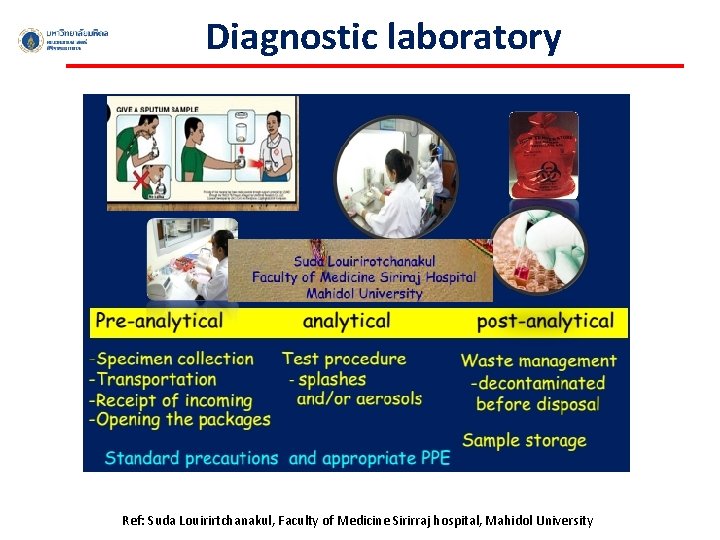
Diagnostic laboratory Ref: Suda Louirirtchanakul, Faculty of Medicine Sirirraj hospital, Mahidol University

Good microbiological practice (GMP) § Do not eat and drink in the lab § Do not apply cosmetic in the lab § Wear gloves and remove gloves properly § Do not touch your stuffs, door knob, any parts of your body with gloves § Clean your lab bench with 70% alcohol every time before&after use § Wash your hands well before leaving § Discard sharp tools, e. g. pipette tip, needle, glass slide, and cover slip, in sharp bin or closed container § Discard infectious stuffs in biohazard bag § Tie long hair back
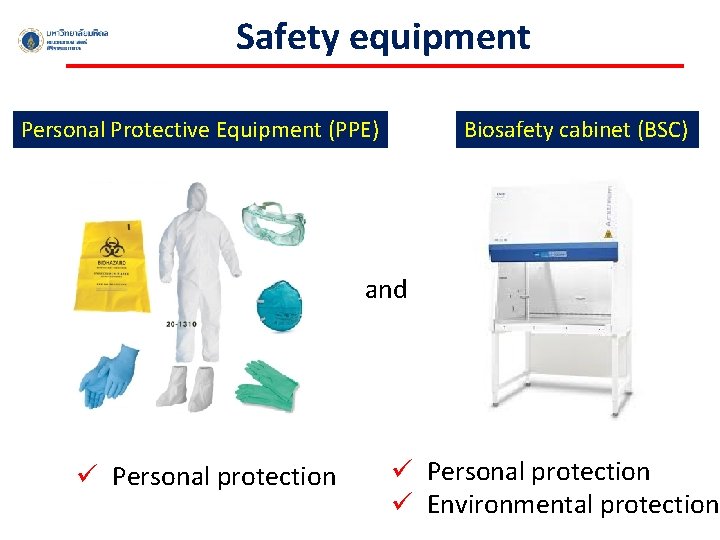
Safety equipment Personal Protective Equipment (PPE) Biosafety cabinet (BSC) and ü Personal protection ü Environmental protection
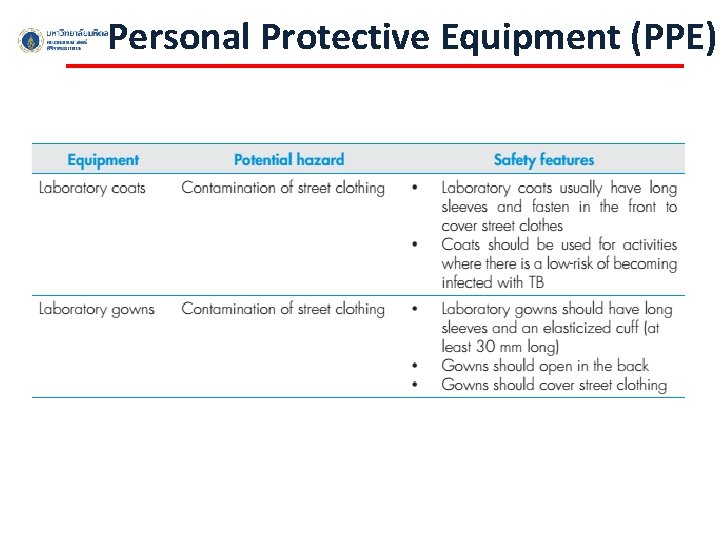
Personal Protective Equipment (PPE)
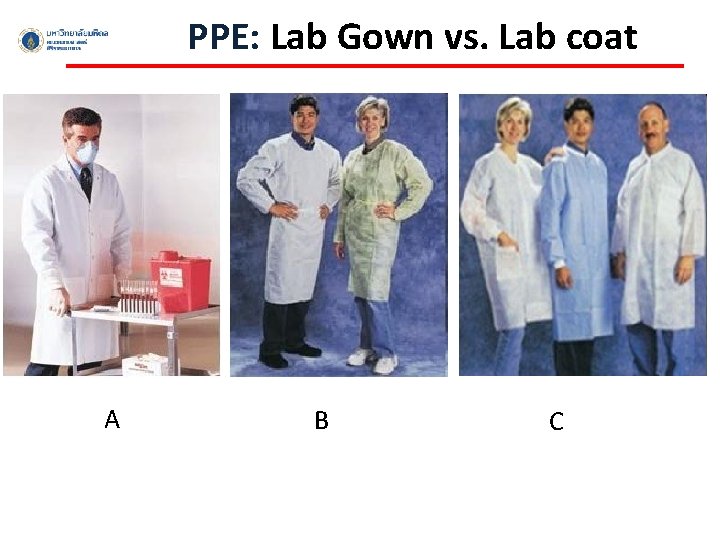
PPE: Lab Gown vs. Lab coat A B C

PPE: respiratory protection surgical mask N-95 mask FFP 2 Particle Filtering Half Mask Powered Air Purifying Respirator (PAPR)
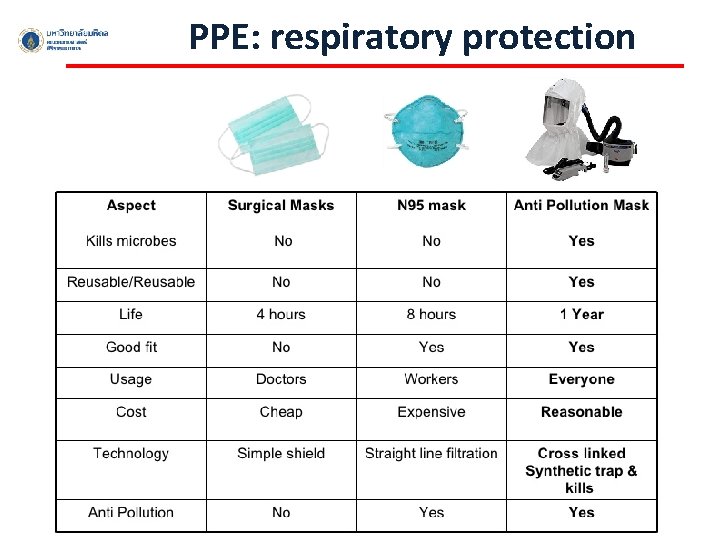
PPE: respiratory protection
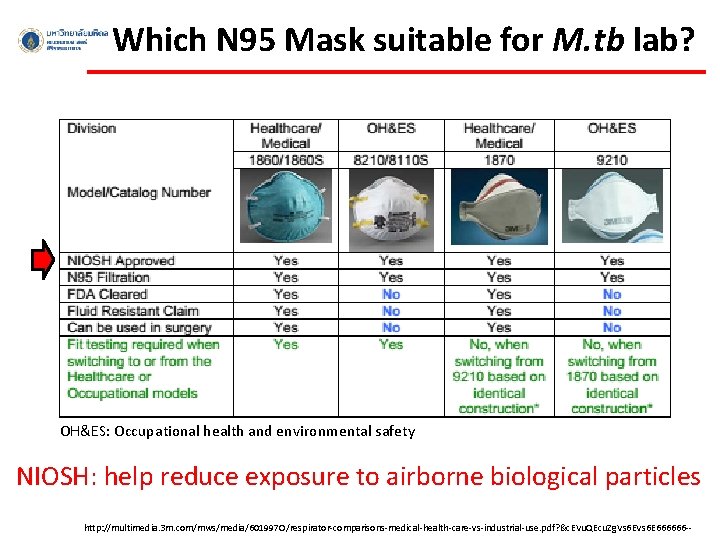
Which N 95 Mask suitable for M. tb lab? OH&ES: Occupational health and environmental safety NIOSH: help reduce exposure to airborne biological particles http: //multimedia. 3 m. com/mws/media/601997 O/respirator-comparisons-medical-health-care-vs-industrial-use. pdf? &c. EVu. QEcu. Zg. Vs 6 E 666666 --

6 steps to wearing N-95 mask http: //1. bp. blogspot. com/-s. LU 8 uj. Do 57 Q/Uche. KCAc. K 2 I/AAAABw 4/LMVBA 7 WBd. AM/s 1600/Wear-N 95 -Mask. jpg

Mask Fit Testing To ensure that the user is properly fitted to use a particular tight fitting respirator ü Wearing the proper mask size and type for the shape of their face and head ü Bitter or sweet odour - Normal Breathing - Deep Breathing - Head side-to-side - Head up and down - Talking ü Every 2 years and recommended annually

PPE: Hand protection Single gloves: BSL 1 &2 Double gloves: BSL 3 Correct size affect technical abilities of worker and may result in unsafe practices

PPE: Hand protection § Gloves must be worn for all procedures that involve or may involve accidental contact, direct contact with sputum, blood, body fluids and other potentially infectious materials. § Disposable gloves must never be reused, and once they have been used they should be discarded with infectious laboratory waste. . § After use, gloves should be removed aseptically and hands washed

How to Safely Remove Disposable Gloves
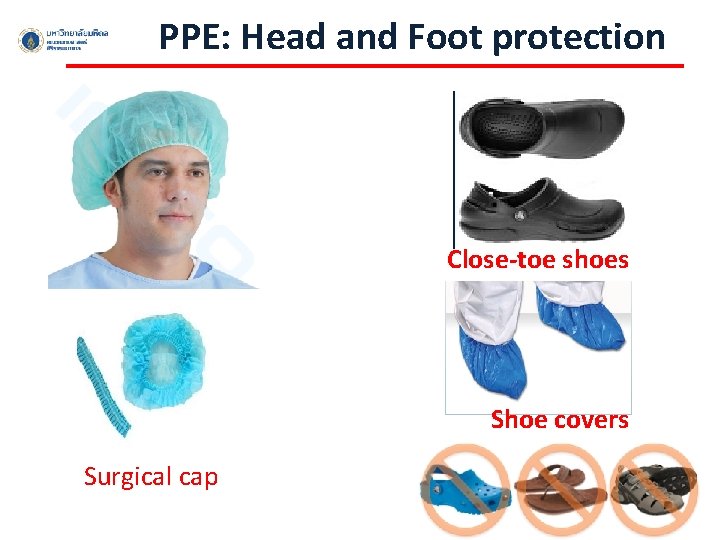
PPE: Head and Foot protection Close-toe shoes Shoe covers Surgical cap

PPE: Safety glasses/Face shield Goggle Face shield In case of wearing eyeglasses, ensure that eye protection is completely cover eyeglasses

Bio-spill kit ü ü ü ü Appropriate, fresh disinfectant HEPA filter masks, protective suit (for large spills) Several pairs of gloves Safety glasses Absorbent material Bio-hazardous waste (autoclave) bags Dust pan & scoop or tongs for broken glass Place in a labeled bag or bucket and keep in areas where biohazards are handled.

Infectious spills (outside a BSC): a major event • • • Everyone should immediately vacate the affected laboratory area The laboratory manager should be informed of the incident immediately, and staff must be prevented from re-entering the laboratory for at least 1 hour Signs should be posted indicating that entry is forbidden during the clean-up procedure Appropriate protective clothing and respiratory protection MUST be worn Anyone who was exposed to the spill should be referred for medical advice; a record should be kept of the incident Tuberculosis laboratory biosafety manual: World Health Organization 2012

Spill clean-up procedure 1. 2. 3. 4. 5. 6. 7. 8. Put on gloves, a protective laboratory gown and respirator. Re-enter the affected area. Cover the spill with cloth or paper towels to contain it. Pour an appropriate disinfectant over the paper towels and the immediate surrounding area (generally, 5% bleach) Apply disinfectant concentrically beginning at the outer margin of the spill and working towards the center. Allow suffi cient time for the disinfectant to act before clearing away any material for disposal. If broken glass or other sharps are involved, use a dustpan or a piece of stiff cardboard to collect the material and place it in a puncture-resistant container for disposal. Place other contaminated material in a sealed bag for appropriate disposal. Clean and disinfect the area of the spill.

Infectious spills (inside BSC) 1. Place absorbent tissue over the spill area, and apply disinfectant solution liberally. 2. If the walls of the BSC have been splashed, clean with a layer of absorbent paper towel liberally soaked in disinfectant solution. 3. Leave affected areas covered with disinfectant for 30 minutes to 1 hour. 4. Carefully collect contaminated sharps material, and place in a puncture-resistant container for disposal. 5. Any equipment or reusable material (eg, centrifuge buckets) that has been splashed should be cleaned with the same disinfectant. 6. Electrical equipment should be checked carefully before it is used; check the integrity of circuit breakers and earth-fault interrupters. 7. Collect other contaminated material in a sealed bag for appropriate disposal. Tuberculosis laboratory biosafety manual: World Health Organization 2012

Cabinet

Biosafety cabinet ü An enclosed, ventilated laboratory workspace for safely working with materials contaminated with (or potentially contaminated with) pathogens ü To protect the laboratory worker and the surrounding environment from pathogens ü All exhaust air is HEPA-filtered as it exits the biosafety cabinet, removing harmful bacteria and viruses.
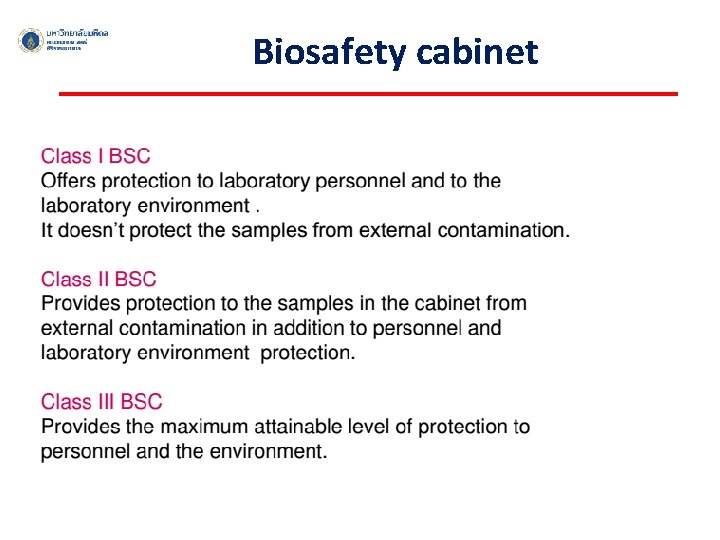
Biosafety cabinet
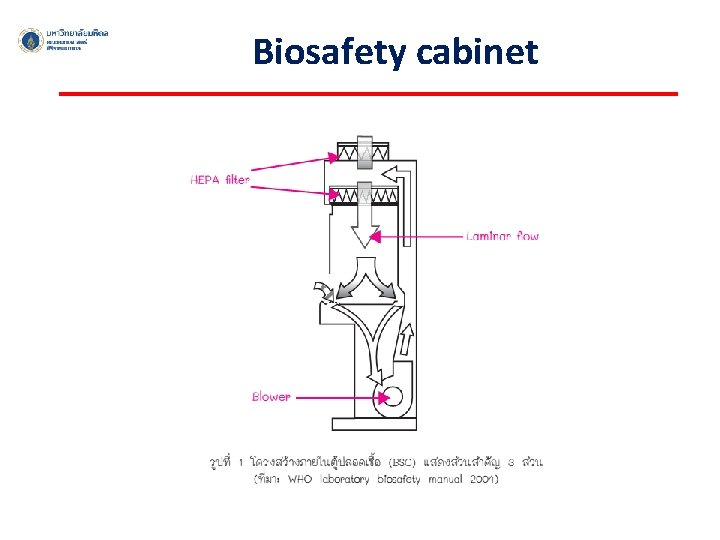
Biosafety cabinet
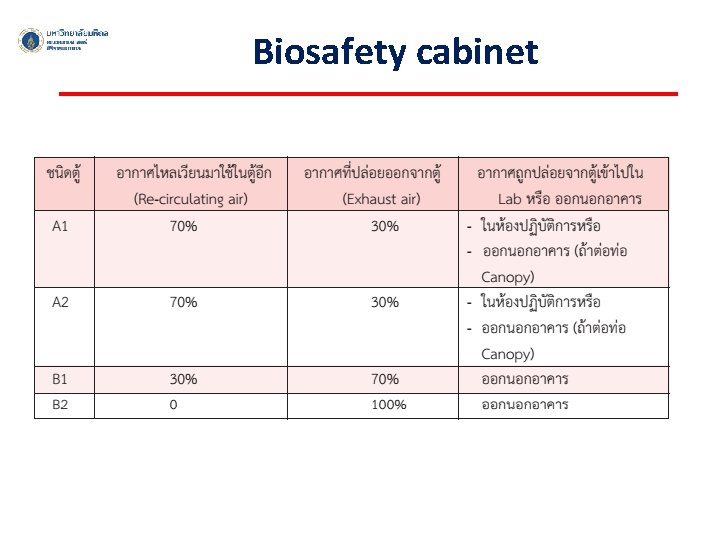
Biosafety cabinet
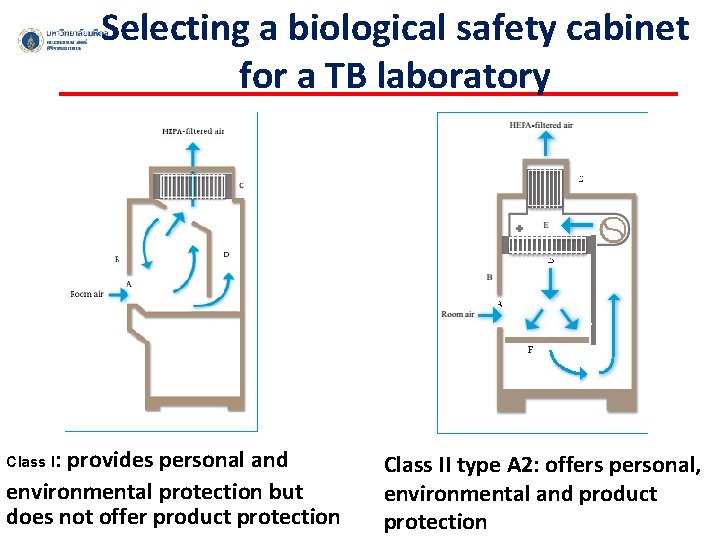
Selecting a biological safety cabinet for a TB laboratory Class I: provides personal and environmental protection but does not offer product protection Class II type A 2: offers personal, environmental and product protection



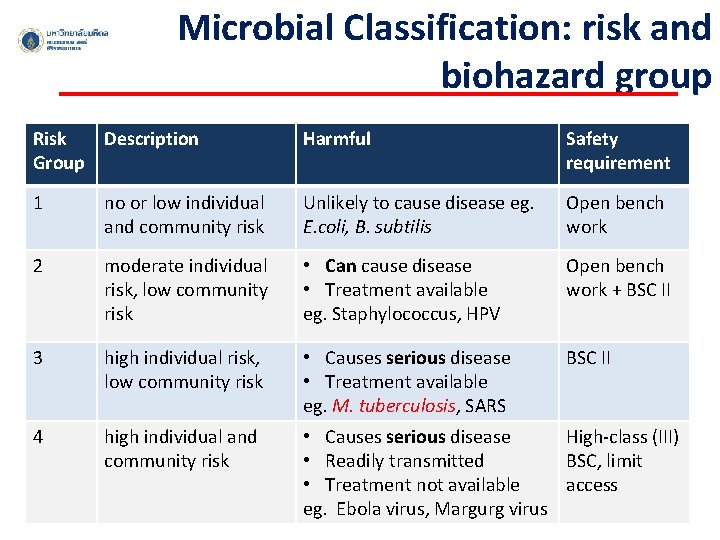
Microbial Classification: risk and biohazard group Risk Description Group Harmful Safety requirement 1 no or low individual and community risk Unlikely to cause disease eg. E. coli, B. subtilis Open bench work 2 moderate individual risk, low community risk • Can cause disease • Treatment available eg. Staphylococcus, HPV Open bench work + BSC II 3 high individual risk, low community risk • Causes serious disease • Treatment available eg. M. tuberculosis, SARS BSC II 4 high individual and community risk • Causes serious disease High-class (III) • Readily transmitted BSC, limit • Treatment not available access eg. Ebola virus, Margurg virus

Microbiology laboratory Working with Mycobacterium tuberculosis necessitates safety practices beyond the basic microbiological practices Laboratory Biosafety Manual: 3 rd edition, 2004, viii + 178 pages, ISBN 92 4 154650 6 Tuberculosis laboratory biosafety manual: World Health Organization 2012
Biosafety: Tuberculosis laboratory ü Assign to the specific work by professional judgement based on an assessment of the risk rather than by automatic assignment of a laboratory biosafety level according to the particular risk group assigned to a pathogenic agent Tuberculosis laboratory biosafety manual: World Health Organization 2012 ü Higher level or lower level of biosafety may be more appropriate based on the specific procedure being performed and other factors ü Minimum requirements , practices, and procedures required to limit infection
Risk assessment and the classification of TB laboratories Considers: § The bacterial load of material (eg. sputum specimens and cultures) and the viability of TB bacilli § Route of transmission § Whether the material handled and the manipulations required for each procedure are likely to generate infectious aerosols § Number of manoeuvres for each technique that may potentially generate aerosols § the workload of the laboratory and individual staff members § the level of experience and the competence of the laboratory’s technician
Classification of TB laboratories Risk level of TB laboratory Low risk Laboratory activities Assessment of risk Direct sputum-smear - Low risk of microscopy; preparation generating infectious of specimens for use in aerosols from an automated nucleic specimens acid amplification test - Low concentration cartridge (such as the of infectious Xpert MTB/RIF assay) particles Tuberculosis laboratory biosafety manual: World Health Organization 2012
Classification of TB laboratories Risk level of TB laboratory Moderate risk Laboratory activities Assessment of risk Processing and concentration of specimens for inoculation on primary culture media; direct DST (eg, on processed sputum) - Moderate risk of generating infectious aerosols from specimens - Low concentration of infectious particles Tuberculosis laboratory biosafety manual: World Health Organization 2012
Classification of TB laboratories Risk level of TB laboratory High risk (TBcontainment laboratory) Laboratory activities Assessment of risk Culture manipulation for - High risk of identifi cation; DST or generating infectious line-probe assays on aerosols from cultured isolates specimens - High concentration of infectious particles DST, drugsusceptibility testing Tuberculosis laboratory biosafety manual: World Health Organization 2012
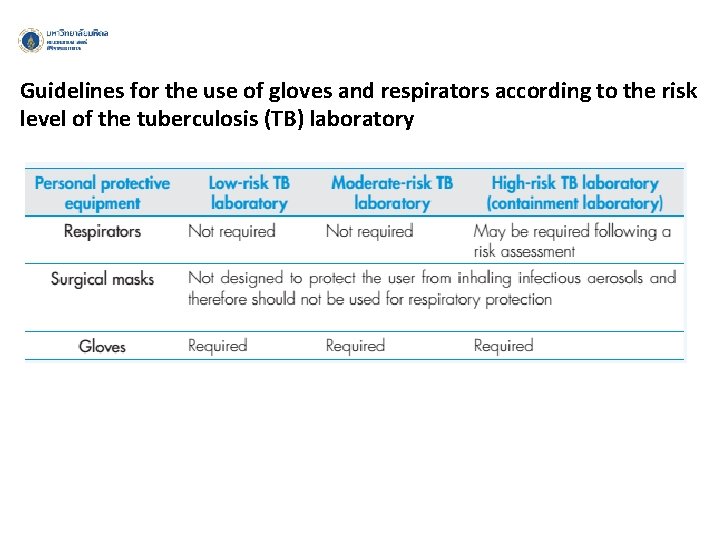
Guidelines for the use of gloves and respirators according to the risk level of the tuberculosis (TB) laboratory
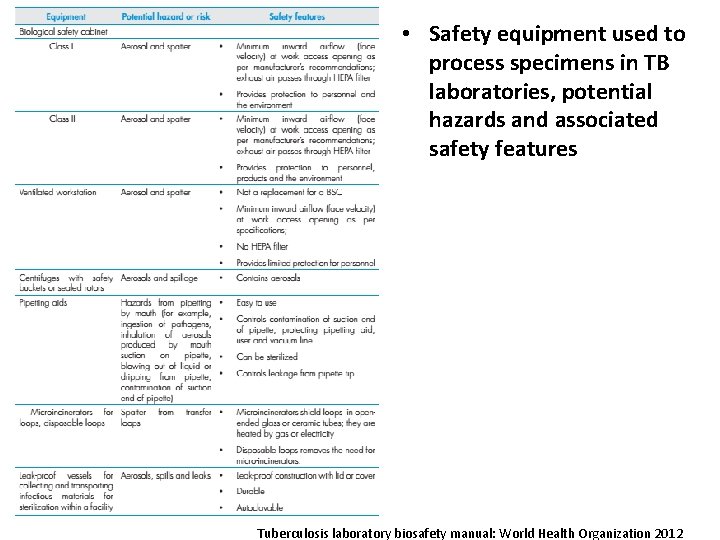
• Safety equipment used to process specimens in TB laboratories, potential hazards and associated safety features Tuberculosis laboratory biosafety manual: World Health Organization 2012
- Slides: 43