Outline Atoms ions and isotopes Molecules Chemical bonds

Outline • • • Atoms, ions, and isotopes Molecules Chemical bonds Special properties of water Acids, bases, and p. H Chemical reactions & energy Kinds of energy Laws of energy Energy quality

DHMO

Why learn chemistry? Chemical basics are at the root of every biological function • Neural impulse (Na/K pump) • Muscular contraction (Ca ion uptake) Percentage (%) of body’s composition THE TOP 10 ELEMENTS FOUND IN YOUR BODY THE “BIG 4” 96% of your body is composed of these 4 elements: Oxygen (65%) Carbon (18. 5%) Hydrogen (9. 5%) Nitrogen (3%) OTHER (4%) Calcium Sulfur Phosphorus Sodium Potassium Chlorine • Trace amounts (less than 0. 1%) of 15 other elements are also found in the body

Figure 2 -3 The highlighted elements are the most abundant elements found in organisms

Atomic Structure • The universe is made up of matter • Matter is made up of atoms – Anything that takes up space and has weight Imagine dividing a gold ring in half, forever • The smallest piece of gold possible is called an atom. If you divided it into smaller pieces, it would no longer be gold. An atom is the smallest basic unit of matter. An element is one type of atom. Atom = uncuttable

Atomic Structure So how do you know if you have an atom of copper, gold or silver? How many protons an atom has determines who it is Cu = 39 Ag = 47 Au = 79 Atomic number is the number of protons Cookium diagnostic A KEY TO THE ELEMENTS ATOMIC NUMBER The number of protons found in the atom’s nucleus ELEMENT SYMBOL Abbreviation of the element ELEMENT NAME ATOMIC MASS Combined mass of the atom’s protons and neutrons

Terms • Atomic number: the number of protons an element possess – What defines an element, every atom of that element has that exact number of protons (or it’s not that element!) – Also indicates the original number of electrons that element has • Atomic mass: the number of protons and neutrons an element possess – The mass of the nucleus (electrons are too tiny to measure)

Figure 2 -2 Mass number (number of protons + neutrons) Atomic number (number of protons)
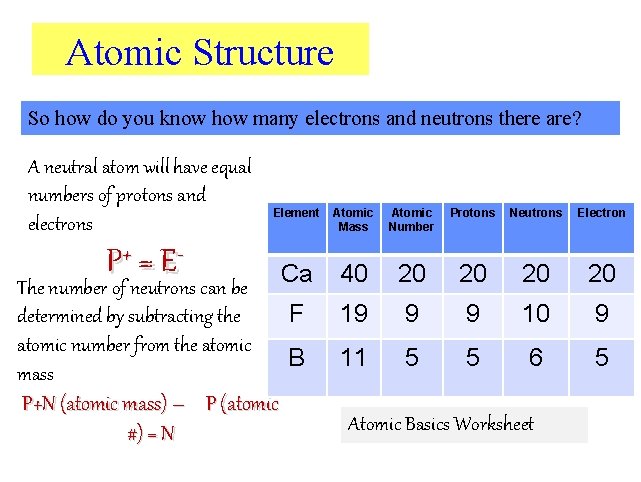
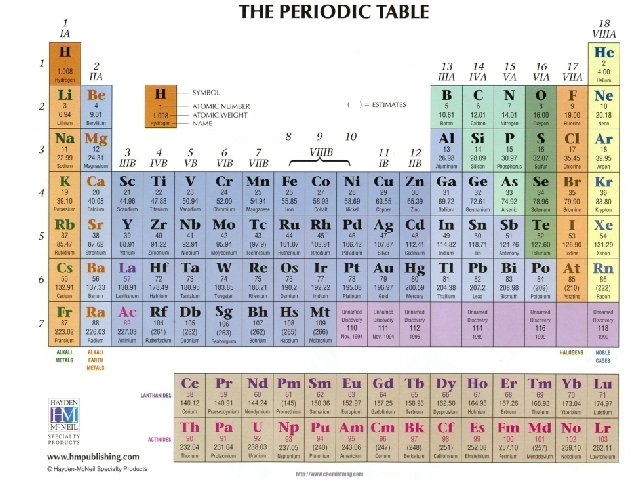
Atomic Structure So how do you know how many electrons and neutrons there are? A neutral atom will have equal numbers of protons and electrons P+ = E - Element The number of neutrons can be Ca F determined by subtracting the atomic number from the atomic B mass P+N (atomic mass) – P (atomic #) = N Atomic Mass Atomic Number Protons Neutrons Electron 40 20 20 19 9 9 10 9 11 5 5 6 5 Atomic Basics Worksheet
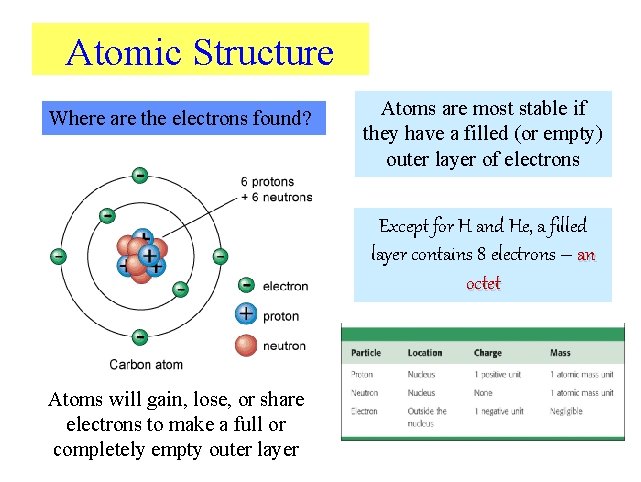
Atomic Structure Where are the electrons found? Atoms are most stable if they have a filled (or empty) outer layer of electrons Except for H and He, a filled layer contains 8 electrons – an octet Atoms will gain, lose, or share electrons to make a full or completely empty outer layer
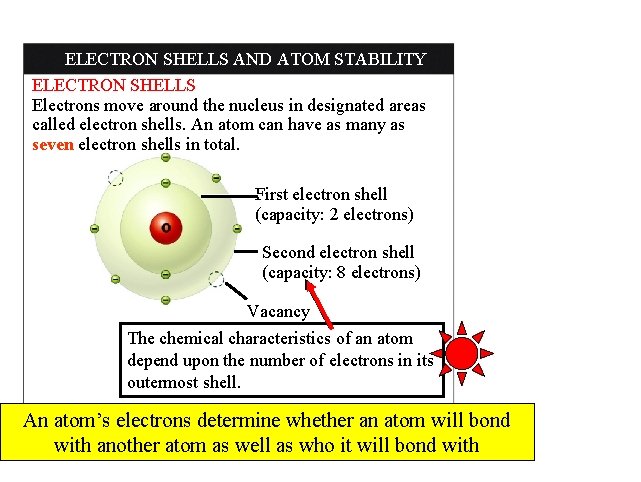
ELECTRON SHELLS AND ATOM STABILITY ELECTRON SHELLS Electrons move around the nucleus in designated areas called electron shells. An atom can have as many as seven electron shells in total. First electron shell (capacity: 2 electrons) Second electron shell (capacity: 8 electrons) Vacancy The chemical characteristics of an atom depend upon the number of electrons in its outermost shell. An atom’s electrons determine whether an atom will bond with another atom as well as who it will bond with
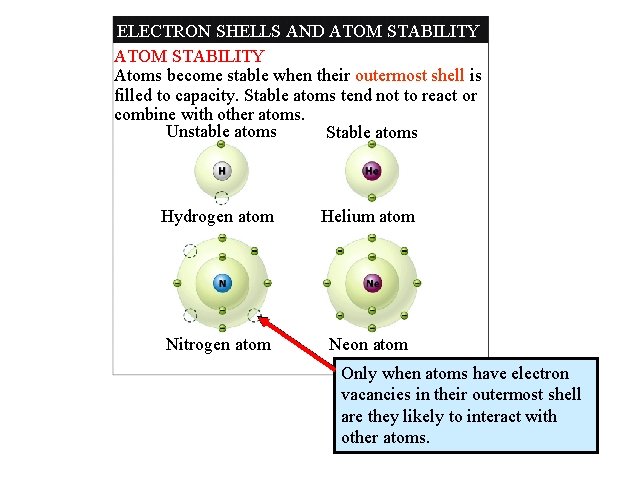
ELECTRON SHELLS AND ATOM STABILITY Atoms become stable when their outermost shell is filled to capacity. Stable atoms tend not to react or combine with other atoms. Unstable atoms Stable atoms Hydrogen atom Helium atom Nitrogen atom Neon atom Only when atoms have electron vacancies in their outermost shell are they likely to interact with other atoms.
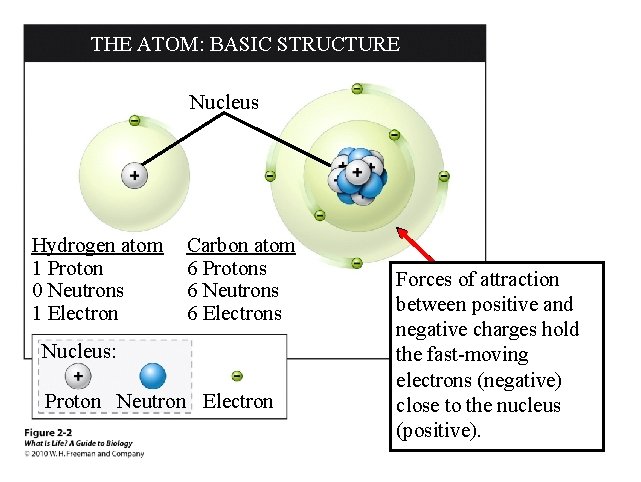
THE ATOM: BASIC STRUCTURE Nucleus Hydrogen atom 1 Proton 0 Neutrons 1 Electron Carbon atom 6 Protons 6 Neutrons 6 Electrons Nucleus: Proton Neutron Electron Forces of attraction between positive and negative charges hold the fast-moving electrons (negative) close to the nucleus (positive).

Chemical Changes When atoms exchange or share electrons, a new product (a compound or molecule) is produced. This is called a chemical change. In a chemical change: • reacting substances form new substances with different compositions and properties. • a chemical reaction takes place. Change Chemica l Melting cheese Milk souring Chemical Change Lab √ √ Ripping paper Bike rusting Physical √ √
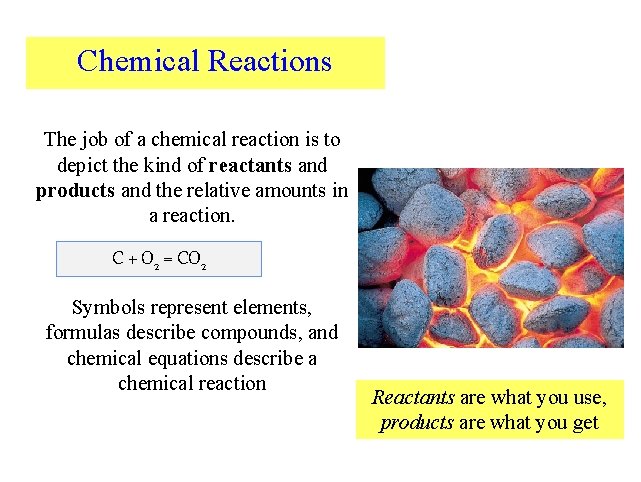
Chemical Reactions The job of a chemical reaction is to depict the kind of reactants and products and the relative amounts in a reaction. C + O 2 = CO 2 Symbols represent elements, formulas describe compounds, and chemical equations describe a chemical reaction Reactants are what you use, products are what you get
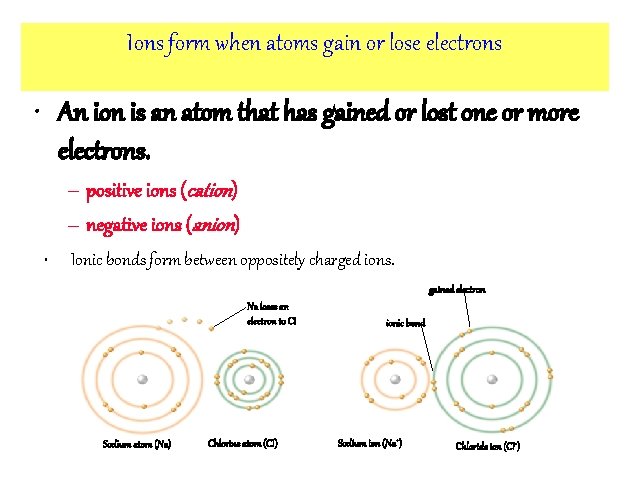
Ions form when atoms gain or lose electrons • An ion is an atom that has gained or lost one or more electrons. – positive ions (cation) – negative ions (anion) • Ionic bonds form between oppositely charged ions. gained electron Na loses an electron to CI Sodium atom (Na) Chlorine atom (CI) ionic bond Sodium ion (Na+) Chloride ion (CI-)

Ions • Atoms can give up electrons or accept electrons from another atom to become charged ions – Atoms will only do this if it makes them more stable – Rule of Thumb: 8 electrons in the outer (valence) energy level makes an atom stable • Isotopes: different forms of the same element that have different numbers of neutrons

Figure 2 -6 ab A sodium ion being formed Loss of electron Cation formation Sodium ion has positive charge A chloride ion being formed Gain of electron Anion formation Chloride ion has negative charge

IONS ARE CHARGED ATOMS An atom that loses one or more electrons becomes positively charged, while an atom that acquires electrons becomes negatively charged. This transfer of electrons is driven by the fact that atoms with full outer electron shells are more stable. Donated electron Na Sodium ion NET CHARGE Cl 11 Protons 10 Electrons Chloride ion 17 Protons 18 Electrons Positive Negative
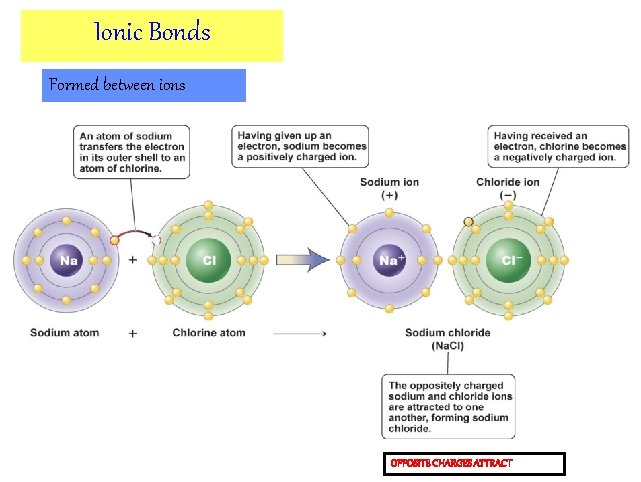
Ionic Bonds Formed between ions OPPOSITE CHARGES ATTRACT

The Human Element Activity • At your groups, decide who is your ‘model’ and dress them up in a trash bag. • You will be assigned an element, decide how many valence electrons it has and inflate and attach the correct number of balloons • Determine your ion, and create a tag to wear • Now BOND!

Atoms combine to form compounds Ionic Compounds – Ions of different charges combine to form ionic compounds (Na+ and Cl- form salt)

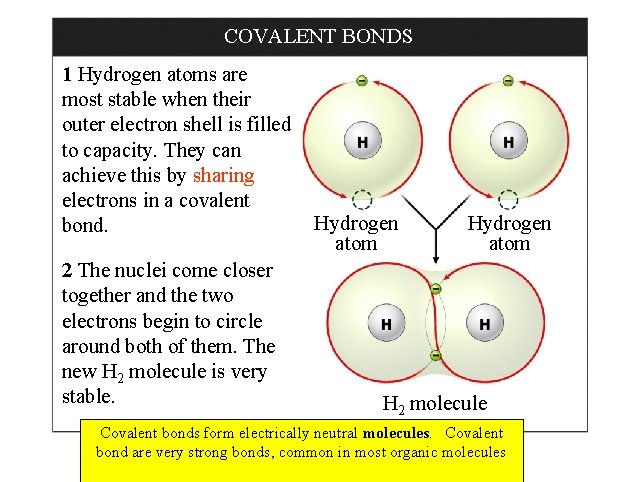
COVALENT BONDS 1 Hydrogen atoms are most stable when their outer electron shell is filled to capacity. They can achieve this by sharing electrons in a covalent bond. 2 The nuclei come closer together and the two electrons begin to circle around both of them. The new H 2 molecule is very stable. Hydrogen atom H 2 molecule Covalent bonds form electrically neutral molecules. Covalent bond are very strong bonds, common in most organic molecules
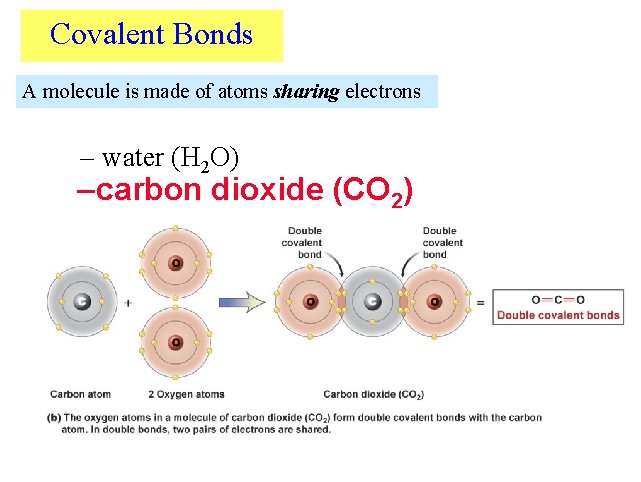
Covalent Bonds A molecule is made of atoms sharing electrons – water (H 2 O) – carbon dioxide (CO 2)
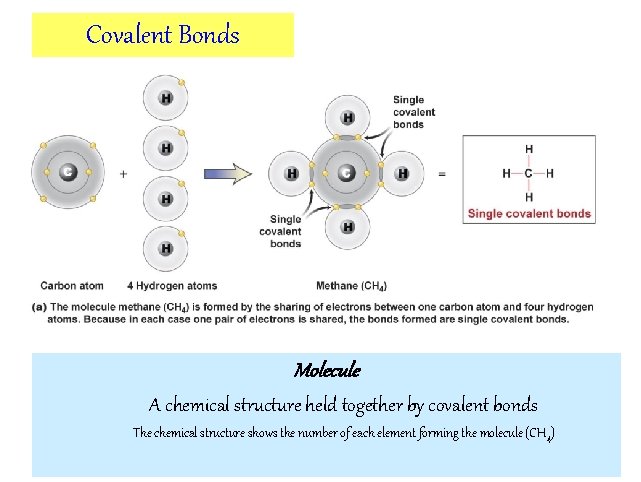
Covalent Bonds Molecule A chemical structure held together by covalent bonds The chemical structure shows the number of each element forming the molecule (CH 4)

Covalent compounds • Often the atoms share the electrons unequally so the molecule (or compound) has an area of positive charge as well as an area of negative charge. – Charged molecules are called polar molecules.

2 IONIC BOND An attraction between two oppositely charged ions, forming a compound. 3 HYDROGEN BOND An attraction between the slightly positively charged hydrogen atom of one molecule and the slightly negatively charged atom of another. Chemical Bonds Rap Chemical Bond SUMMARY: THREE TYPES OF BONDS Strongest H 2 molecule Bond Strength 1 COVALENT BOND A strong bond formed when atoms share electrons in order to become more stable, forming a molecule. Na. Cl compound Weakest H 2 O

Balloon Buddy Your challenge: get the balloon to kiss you Simulation Ions (charged atoms) follow the rule that opposites attract Static electricity is a reflection of the difference in charges between objects
Concept map • Using your vocabulary journal build a concept map to show connections. • Atom • Element • Compound • Ionic bond • covalent bond • molecule
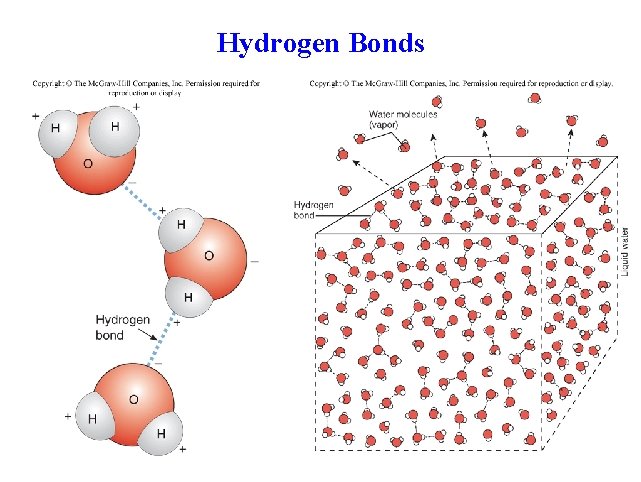
Hydrogen Bonds

Polar molecules • Polar molecules can be attracted to one another. • The bond that holds these molecules together is called a hydrogen bond. – Hydrogen bonds are very weak; heat and changes in p. H can break these bonds.




Elixir of Life • Special properties of water – cohesion & adhesion • surface tension, capillary action – good solvent • many molecules dissolve in H 2 O • hydrophilic vs. hydrophobic – lower density as a solid • ice floats! – high specific heat • water stores heat – high heat of vaporization • heats & cools slowly

Cohesion & Adhesion • H bonding between H 2 O molecules is cohesion – water is “sticky” • surface tension • drinking straw • H bonding between H 2 O & other substances is adhesion – capillary action – meniscus – water climbs up paper towel or cloth Can you suck sugar up a straw?
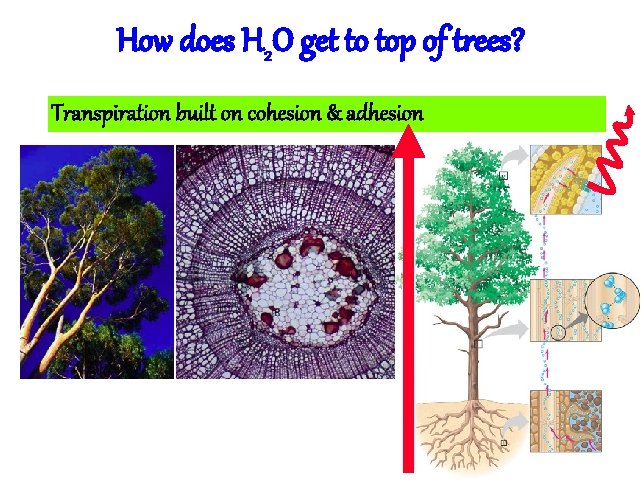
How does H 2 O get to top of trees? Transpiration built on cohesion & adhesion

Water is the solvent of life • Polarity makes H 2 O a good solvent – polar H 2 O molecules surround + & – ions – solvents dissolve solutes creating solutions

Do you dissolve in water? • Hydrophilic – substances have attraction to H 2 O – polar or non-polar?

Or don’t you? • Hydrophobic – substances that don’t have an attraction to H 2 O – polar or non-polar? fat (triglycerol)

The special case of ice • Most (all? ) substances are more dense when they are solid H bonds form a crystal in water
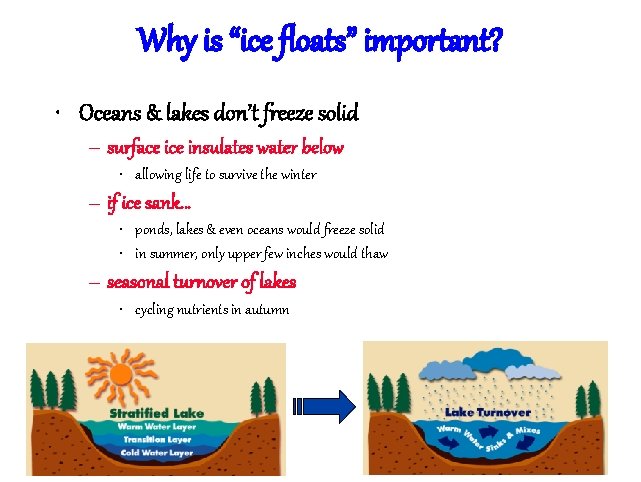
Why is “ice floats” important? • Oceans & lakes don’t freeze solid – surface insulates water below • allowing life to survive the winter – if ice sank… • ponds, lakes & even oceans would freeze solid • in summer, only upper few inches would thaw – seasonal turnover of lakes • cycling nutrients in autumn

Evaporative cooling Heat of vaporization Organisms rely on heat of vaporization to remove body heat
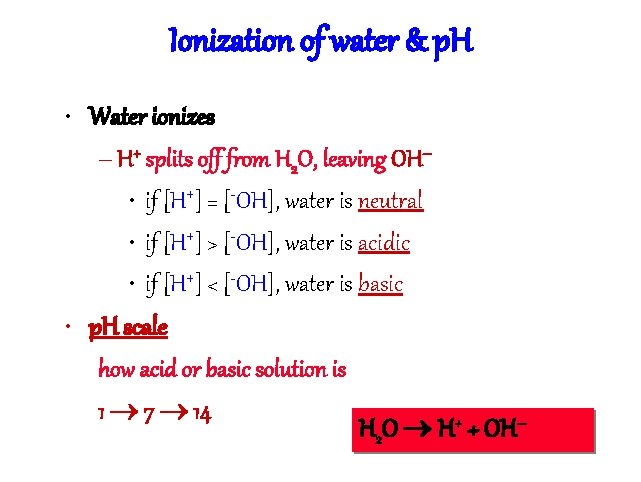
Ionization of water & p. H • Water ionizes – H+ splits off from H 2 O, leaving OH– • if [H+] = [-OH], water is neutral • if [H+] > [-OH], water is acidic • if [H+] < [-OH], water is basic • p. H scale how acid or basic solution is 1 7 14 H 2 O H+ + OH–
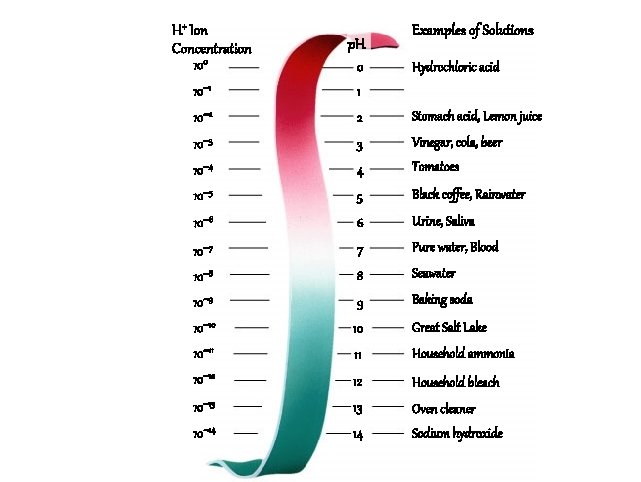
p. H Scale H+ Ion Concentration 100 0 Examples of Solutions Hydrochloric acid 10– 1 1 10– 2 2 Stomach acid, Lemon juice 10– 3 3 Vinegar, cola, beer 10– 4 4 Tomatoes 10– 5 5 Black coffee, Rainwater 10– 6 6 Urine, Saliva 10– 7 7 Pure water, Blood 10– 8 8 Seawater 10– 9 9 Baking soda 10– 10 10 Great Salt Lake 10– 11 11 Household ammonia 10– 12 12 Household bleach 10– 13 13 Oven cleaner 10– 14 14 Sodium hydroxide

Buffers & cellular regulation • p. H of cells must be kept ~7 – p. H affects shape of molecules – shape of molecules affect function – p. H affects cellular function • Control p. H by buffers • donate H+ when [H+] falls • absorb H+ when [H+] rises p. H – reservoir of H+ 9 8 7 6 5 4 3 2 1 0 Buffering range 0 1 2 3 4 Amount of base added 5

Energy • Defined as the capacity to do work – Potential: stored energy – Kinetic: the energy of motion • Radiation: the transmission of energy through space • Electromagnetic radiation: different types based on wavelength – X ray, gamma ray, microwaves, etc

High-quality matter vs. low-quality matter • High-quality matter is: – Organized – Concentrated – Found near the earth’s surface – Has great potential for use
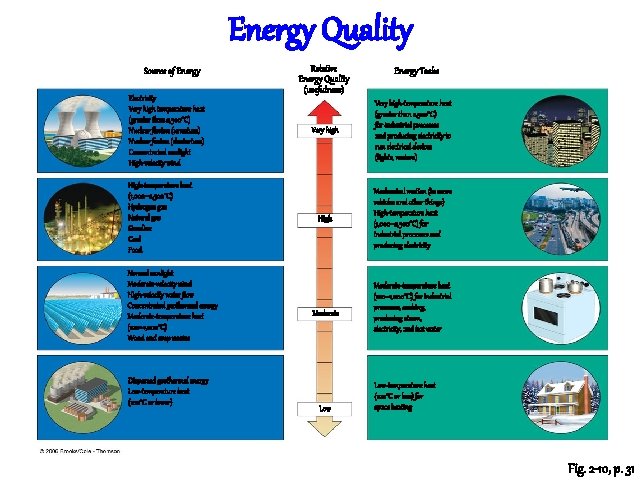
Energy Quality Source of Energy Electricity Very high temperature heat (greater than 2, 500°C) Nuclear fission (uranium) Nuclear fusion (deuterium) Concentrated sunlight High-velocity wind High-temperature heat (1, 000– 2, 500°C) Hydrogen gas Natural gas Gasoline Coal Food Normal sunlight Moderate-velocity wind High-velocity water flow Concentrated geothermal energy Moderate-temperature heat (100– 1, 000°C) Wood and crop wastes Dispersed geothermal energy Low-temperature heat (100°C or lower) Relative Energy Quality (usefulness) Energy Tasks Very high-temperature heat (greater than 2, 500°C) for industrial processes and producing electricity to run electrical devices (lights, motors) High Mechanical motion (to move vehicles and other things) High-temperature heat (1, 000– 2, 500°C) for industrial processes and producing electricity Moderate Low Moderate-temperature heat (100– 1, 000°C) for industrial processes, cooking, producing steam, electricity, and hot water Low-temperature heat (100°C or less) for space heating Fig. 2 -10, p. 31


Figure 04_09

Figure 04_10

Balancing Reactions Due to the Law of Conservation of Mass: matter cannot be created nor destroyed. An equation must be balanced (it must have the same number and kinds of atoms both before and after a reaction. Think of a recipe without any measurements Reaction Rate Demo When balancing a chemical reaction you may add coefficients in front of the compounds to balance the reaction, but you may not change the subscripts. Changing the subscripts changes the compound. Subscripts are determined by the valence electrons (charges for ionic or sharing for covalent)

• Physical change: no change in chemical composition (cutting matter does not alter it chemically) • Chemical change: composition of the elements or atoms are altered • Shorthand equations are used to illustrate a chemical reaction C 6 H 12 O 6 + O 2 CO 2 + H 2 O
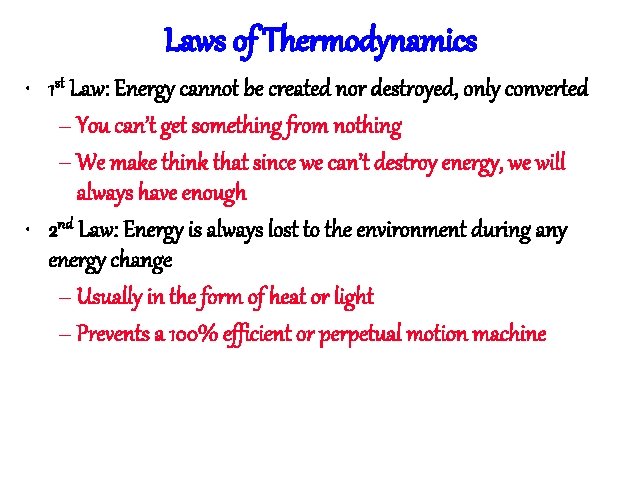
Laws of Thermodynamics • 1 st Law: Energy cannot be created nor destroyed, only converted – You can’t get something from nothing – We make think that since we can’t destroy energy, we will always have enough • 2 nd Law: Energy is always lost to the environment during any energy change – Usually in the form of heat or light – Prevents a 100% efficient or perpetual motion machine
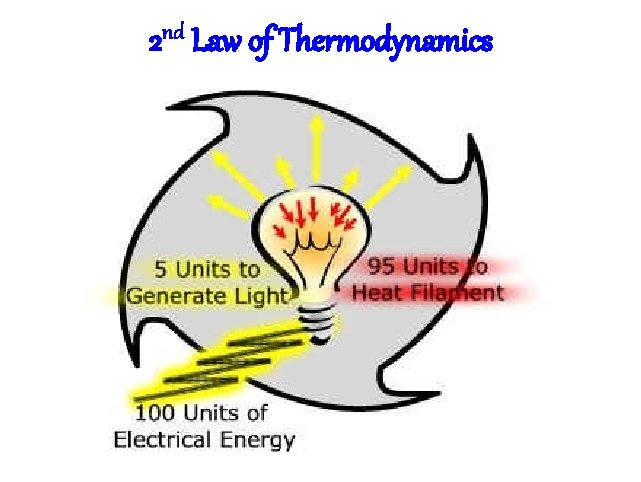
2 nd Law of Thermodynamics

Conservation Laws • The only time matter is converted to energy or visa versa is during a nuclear reaction (which do not occur spontaneously here on earth) – When there is a transfer of protons, the element changes into a different type of atom (a new element) – This occurs with unstable isotopes • Emit gamma rays, alpha or beta particles

Isotopes Going back to those neutrons… Elements with the same number of protons but different number of neutrons are called isotopes

Isotopes Neutron + + + Electrons Nucleus + + + Proton Nucleus Carbon-12 Neutrons Protons Electrons As istopes decay, the release nuclear particles at a rate called a half life Nucleus Proton + 6 6 6 + + + Carbon-14 Neutrons Protons Electrons Neutron Electrons + + 8 6 6 Nucleus
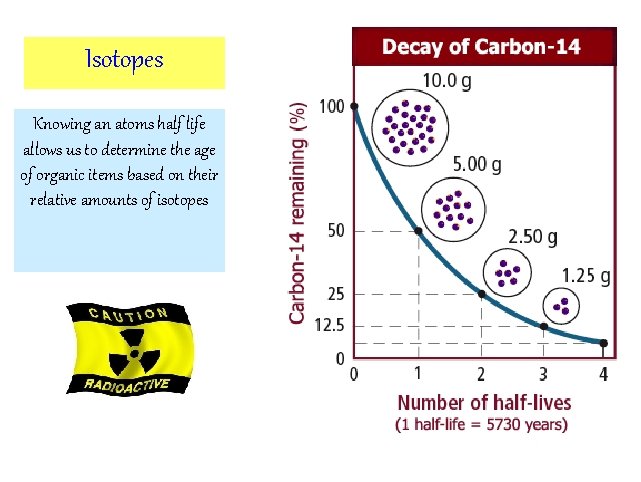
Isotopes Knowing an atoms half life allows us to determine the age of organic items based on their relative amounts of isotopes

Radioactive Decay • Each type of radioisotope decays at a characteristic rate to a different isotope (this is it’s half-life) – Half life: time it takes for ½ of a sample to decay, continues through many different isotopes until a nonradioactive isotope is formed – An isotope’s ½ life cannot be changed by temp, pressure, or chemical rxns

Nuclear Fission • Large nuclei of isotopes are split into 2 lighter nuclei when struck by neutrons – Each collision (fission) releases more neutrons which can then strike more nuclei = chain reaction – Enormous amounts of energy are released (power of nuclear plants, atomic bombs)


Nuclear Fusion • Two small nuclei from different isotopes collide under extreme temps to form a second, larger isotope • Not as well understood – Occurs at temperatures not practical on earth – Source of energy for the sun

Laws of Thermodynamics • 1 st Law: Energy cannot be created nor destroyed, only converted – You can’t get something from nothing – We make think that since we can’t destroy energy, we will always have enough • 2 nd Law: Energy is always lost to the environment during any energy change – Usually in the form of heat or light – Prevents a 100% efficient or perpetual motion machine

2 nd Law of Thermodynamics

Chemical Symbols The subscripts tell you how many atoms of a particular element are in a compound. The coefficient tells you about the number of molecules of the compound.
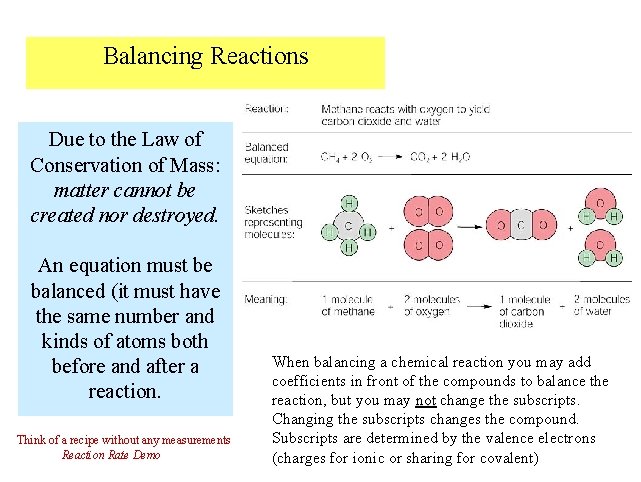
Balancing Reactions Due to the Law of Conservation of Mass: matter cannot be created nor destroyed. An equation must be balanced (it must have the same number and kinds of atoms both before and after a reaction. Think of a recipe without any measurements Reaction Rate Demo When balancing a chemical reaction you may add coefficients in front of the compounds to balance the reaction, but you may not change the subscripts. Changing the subscripts changes the compound. Subscripts are determined by the valence electrons (charges for ionic or sharing for covalent)

Elements & their valence shells Elements in the same row have the same number of shells Moving from left to right, each element has a sequential addition of electrons (& protons)

Elements & their valence shells Elements in the same column have the same valence & similar chemical properties
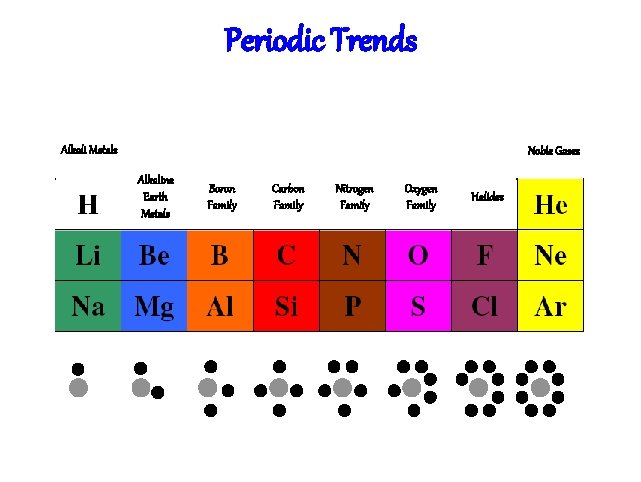
Periodic Trends Alkali Metals Noble Gases Alkaline Earth Metals Boron Family Carbon Family Nitrogen Family Oxygen Family Halides
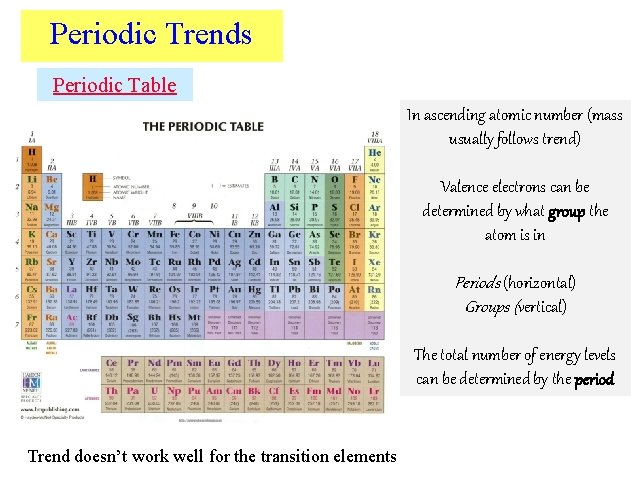
Periodic Trends Periodic Table In ascending atomic number (mass usually follows trend) Valence electrons can be determined by what group the atom is in Periods (horizontal) Groups (vertical) The total number of energy levels can be determined by the period Trend doesn’t work well for the transition elements
Molecules of Life • Put C, H, O, N together in different ways to build living organisms • What are bodies made of? – carbohydrates • sugars & starches – proteins – fats (lipids) – nucleic acids • DNA, RNA

Don’t forget water • Water – 65% of your body is H 2 O – water is inorganic • doesn’t contain carbon • Rest of you is made of carbon molecules – organic molecules • • carbohydrates proteins fats nucleic acids 5

Carbon atoms have unique bonding properties. • Carbon-based molecules have three general types of structures. – straight chain – branched chain – ring
The molecules of life • Organic molecules contain carbon – Carbohydrates, lipids, proteins, and nucleic acids are 4 types. • Inorganic molecules constitute nonliving matter, but they are still important to us. – Salts, water, acids, bases, and carbon dioxide are examples.
- Slides: 78