Outcomes of 10 312 Patients Treated with Everolimuseluting


![Procedural parameters Patients Total device length implanted (mm), median [IQR] Minimum device diameter patient Procedural parameters Patients Total device length implanted (mm), median [IQR] Minimum device diameter patient](https://slidetodoc.com/presentation_image_h2/487d6beb2fe8ca70dc58ed6479e2ed65/image-8.jpg)

- Slides: 16

Outcomes of 10. 312 Patients Treated with Everolimus-eluting Bioresorbable Scaffolds During Daily Clinical Practice: Results from the European Absorb Consortium Holger M. Nef Jens Wiebe, Nick West, Andreas Baumbach, Didier Carrie, Eduardo Pinar Bermudez, Guillaume Cayla, Felipe Hernandez, Jose M de la Torre Hernandez, René Koning, Bruno Loi, Elisabetta Moscarella, Giuseppe Tarantini, Azfar Zaman, Christiane Lober, Thomas Riemer, Stephan Achenbach, Christian W. Hamm on behalf of the European ABSORB Consortium (EAC) investigators University of Giessen, Germany

Disclosure Statement of Financial Interest I, (Holger M. Nef) disclose the following potential conflicts of interests: Research Grant: Abbott Vascular, Elixir Medical Speaker‘s fee: Abbott Vascular, Elixir Medical

Background • The European ABSORB Consortium (EAC) was designed to monitor usage and outcomes of bioresorbable scaffold (BVS) implantation in everyday interventional practice in European countries. • The EAC is the largest registry of BVS globally and allows accurate assessment of low-frequency clinical outcome parameters including device thrombosis and restenosis.

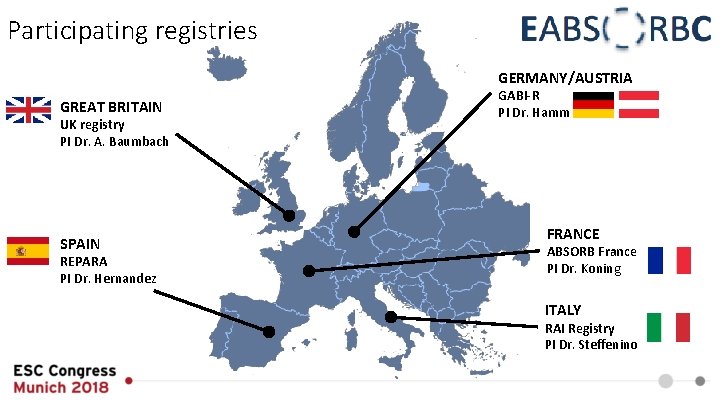
Participating registries GERMANY/AUSTRIA GREAT BRITAIN UK registry PI Dr. A. Baumbach SPAIN REPARA PI Dr. Hernandez GABI-R PI Dr. Hamm FRANCE ABSORB France PI Dr. Koning ITALY RAI Registry PI Dr. Steffenino

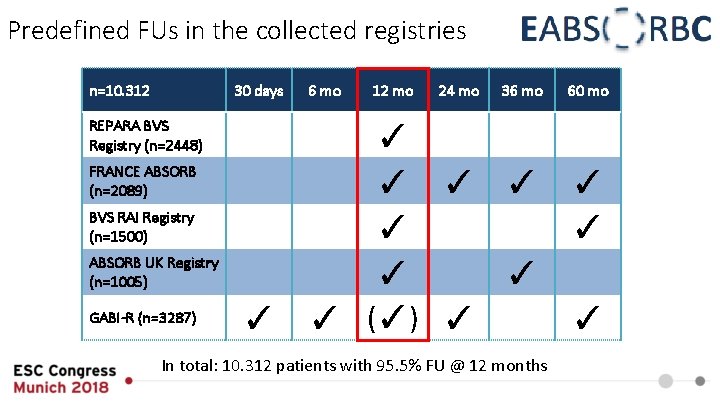
Predefined FUs in the collected registries n=10. 312 30 days REPARA BVS Registry (n=2448) FRANCE ABSORB (n=2089) BVS RAI Registry (n=1500) ABSORB UK Registry (n=1005) GABI-R (n=3287) ✓ 6 mo 12 mo 24 mo 36 mo ✓ ✓ ✓ ✓ (✓) ✓ In total: 10. 312 patients with 95. 5% FU @ 12 months 60 mo ✓ ✓ ✓

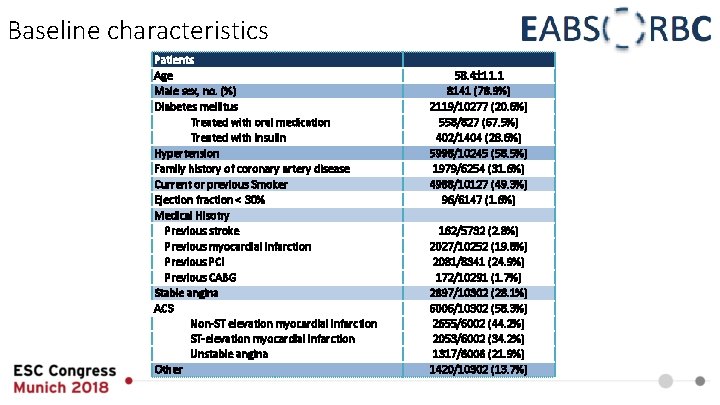
Baseline characteristics Patients Age Male sex, no. (%) Diabetes mellitus Treated with oral medication Treated with insulin Hypertension Family history of coronary artery disease Current or previous Smoker Ejection fraction < 30% Medical Hisotry Previous stroke Previous myocardial infarction Previous PCI Previous CABG Stable angina ACS Non-ST elevation myocardial infarction ST-elevation myocardial infarction Unstable angina Other 58. 4 11. 1 8141 (78. 9%) 2119/10277 (20. 6%) 558/827 (67. 5%) 402/1404 (28. 6%) 5998/10245 (58. 5%) 1979/6254 (31. 6%) 4988/10127 (49. 3%) 96/6147 (1. 6%) 162/5732 (2. 8%) 2027/10252 (19. 8%) 2081/8341 (24. 9%) 172/10291 (1. 7%) 2897/10302 (28. 1%) 6006/10302 (58. 3%) 2655/6002 (44. 2%) 2053/6002 (34. 2%) 1317/6006 (21. 9%) 1420/10302 (13. 7%)

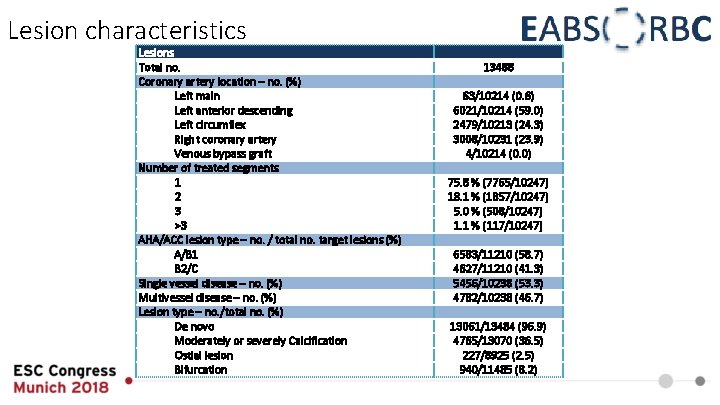
Lesion characteristics Lesions Total no. Coronary artery location – no. (%) Left main Left anterior descending Left circumflex Right coronary artery Venous bypass graft Number of treated segments 1 2 3 >3 AHA/ACC lesion type – no. / total no. target lesions (%) A/B 1 B 2/C Single vessel disease – no. (%) Multivessel disease – no. (%) Lesion type – no. /total no. (%) De novo Moderately or severely Calcification Ostial lesion Bifurcation 13488 63/10214 (0. 6) 6021/10214 (59. 0) 2479/10213 (24. 3) 3008/10231 (23. 9) 4/10214 (0. 0) 75. 8 % (7765/10247) 18. 1 % (1857/10247) 5. 0 % (508/10247) 1. 1 % (117/10247) 6583/11210 (58. 7) 4627/11210 (41. 3) 5456/10238 (53. 3) 4782/10238 (46. 7) 13061/13484 (96. 9) 4765/13070 (36. 5) 227/8925 (2. 5) 940/11485 (8. 2)
![Procedural parameters Patients Total device length implanted mm median IQR Minimum device diameter patient Procedural parameters Patients Total device length implanted (mm), median [IQR] Minimum device diameter patient](https://slidetodoc.com/presentation_image_h2/487d6beb2fe8ca70dc58ed6479e2ed65/image-8.jpg)
Procedural parameters Patients Total device length implanted (mm), median [IQR] Minimum device diameter patient (mm), median [IQR] 23. 0 [18. 0, 35. 0] 3. 0 [2. 5, 3. 5] Access route – no. / total (%) Femoral Radial Imaging - no. / total (%) Intravascular ultrasound Optical coherence tomography Patient with BVS treated – no. (%) Patient with BVS and DES treated - no. (%) Predilation of lesion – no. (%) Max. balloon diameter of predilation (mm), median [IQR] 274/9640 (2. 8) 450/9640 (4. 7) 8463/10282 (82. 3) 1819/10282 (17. 7) 12068/13382 (90. 2) 3. 0 [2. 5, 3. 0] Postdilation - no. (%) Max balloon diameter of postdilation (mm), median [IQR] 9858/13365 (73. 8) 3. 5 [3. 0, 3. 5] Medication at discharge Aspirin P 2 Y 12 inhibitor 7904/8232 (96. 0) 7841/8230 (95. 3) 2139/6675 (32. 0) 4523/6675 (67. 8)

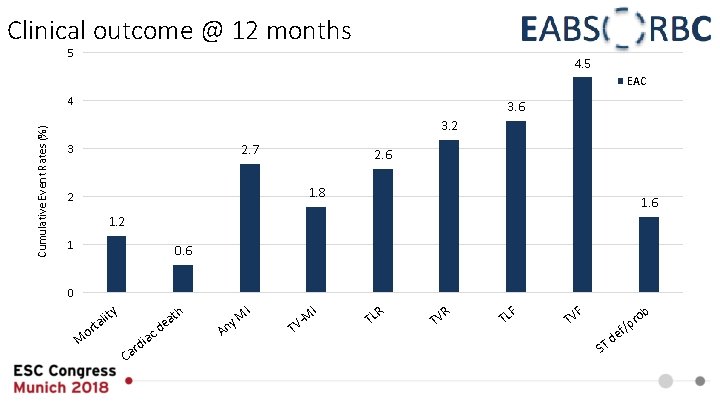
Clinical outcome @ 12 months 5 4. 5 EAC Cumulative Event Rates (%) 4 3. 6 3. 2 2. 7 3 2. 6 1. 8 2 1. 6 1. 2 1 0. 6 0 ty M li ta r o r Ca c dia d I I h t ea A M ny T M V- R TL R TV TL F F ob TV d ST e r f/p

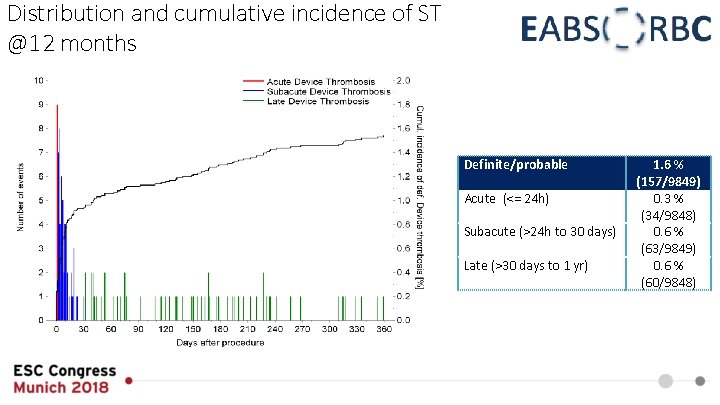
Distribution and cumulative incidence of ST @12 months Definite/probable Acute (<= 24 h) Subacute (>24 h to 30 days) Late (>30 days to 1 yr) 1. 6 % (157/9849) 0. 3 % (34/9848) 0. 6 % (63/9849) 0. 6 % (60/9848)

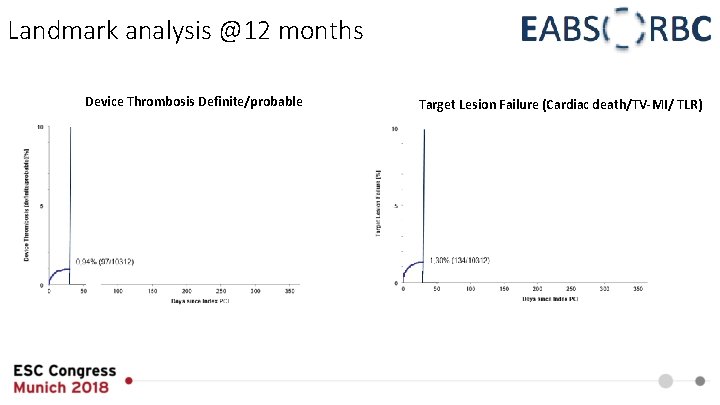
Landmark analysis @12 months Device Thrombosis Definite/probable Target Lesion Failure (Cardiac death/TV-MI/ TLR)

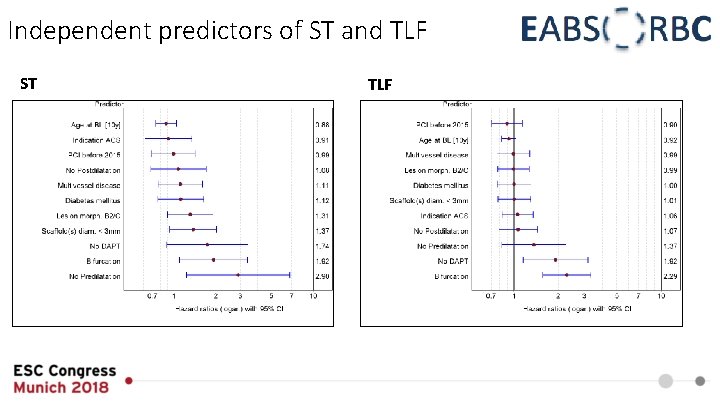
Independent predictors of ST and TLF ST TLF

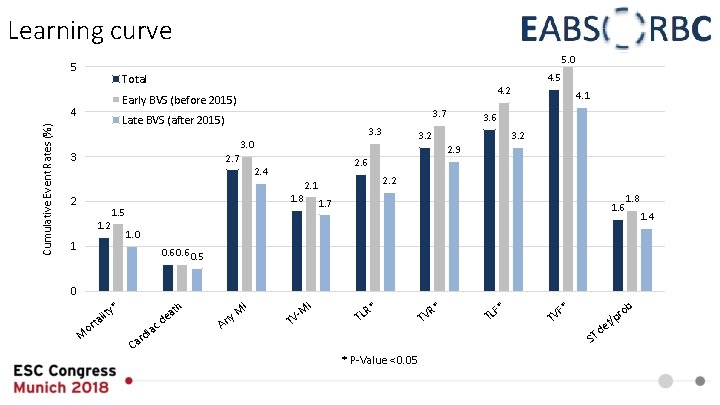
Learning curve 5. 0 5 4. 2 Early BVS (before 2015) 4 Cumulative Event Rates (%) 4. 5 Total 3. 7 Late BVS (after 2015) 3. 3 3 2. 6 2. 4 2. 2 2. 1 1. 8 1. 7 1. 5 1. 2 3. 2 2. 9 2. 7 2 3. 6 3. 2 3. 0 4. 1 1. 6 1. 8 1. 0 1 0. 6 0. 5 0 * o M I th lity a t r iac C d ar a de A M ny I M TV R* TL * P-Value <0. 05 R* TV F* TL * F TV ob d ST e r f/p 1. 4

Summary • In the EAC low clinical event rates could be documented after 12 months • ST rate was lower than expected from RCT • Pre- and postdilatation were performed frequently and thoroughly • Independent predictors of ST at 12 months were identified to be lack of predilatation and bifurcation lesion • The landmark analysis demonstrated clustering of events within the first 30 days without an exceptional rate of ST beyond that point • A learning curve for the adapted implantation technique was associated with lower rates of TLF and TVF

Conclusion • The EAC demonstrates favorable real-world clinical outcome data for BVS, with TLF comparable to data available for secondgeneration DES. • Clinical outcome after treatment with everolimus-eluting bioresorbable scaffold might be improved by specific implantation protocols and newer iterations of bioresorbable scaffolds

Thank you! René Koning, France Elisabetha Moscarella, Italy Jochen Wöhrle, Germany Didier Carre, France Guiseppe Tarantini, Italy Gerd Richardt, Germany Guilaume Cayla, France Alfonoso Lelasi, Italy Ralf Zahn, Germany Patrick Motreff, France Bruno Loi, Italy Julina Mehilli, Germany Vincent Bataille, France Andreas Baumbauch, UK Axel Schmermund, Germany Gilard Martine, France Nick West, UK Johannes Kastner, Austria Nicole Naccache, France Azfar Zaman, UK Alexander Neumer, Germany Felipe Hernandez, Spain Christian Hamm, Germany Christiane Lober, Germany Jose M de la Torre Hernandez, Spain Stephan Achenbach, Germany Alexandra Bernhardt, Germany Juan Jose Cruz, Spain Jens Wiebe, Germany Wolfgang Witsch, Germany Eduardo Pinar Bermudez, Spain Tommaso Gori, Germany Thomas Pfannebecker, Germany Bernardo Cortese, Italy Christoph Naber, Germany Els Boone, Belgium Guiseppe Steffenino, Italy Till Neumann, Germany Adriaan Potgieter, Belgium