Outcomes in Elderly Patients Undergoing PCI Treated with




- Slides: 21

Outcomes in Elderly Patients Undergoing PCI Treated with Bivalirudin Monotherapy versus Glycoprotein IIb/IIIa Inhibitors with Heparin or LMWH: Results from the Randomized ACUITY Trial Karen P. Alexander, E. Magnus Ohman, Michel E. Bertrand, Frederic Feit, Charles V. Pollack Jr, James Hoekstra, Bernard J. Gersh, Harvey D. White, Gregg W. Stone for the ACUITY Investigators TCT Presentation October 2006

Disclosures n Research Funding (Minor): Schering Plough, BMS, Amgen, CV Therapeutics n Speakers Bureau: Pfizer TCT Presentation October 2006

Background n Elderly patients presenting with NSTE ACS are at high risk for recurrent ischemic events l Use of antithrombotic therapy and an early invasive strategy are beneficial n Elderly patients are at high risk for bleeding with antithrombotic therapy and catheter interventions l Major bleeding is associated with adverse outcomes n Therapy for NSTEACS has become multi-tiered, particularly in pts undergoing PCI TCT Presentation October 2006

Bivalirudin n Bivalirudin is a direct thrombin inhibitor with certain advantages l l l n Circulating and clot bound thrombin, no requirement for AT III, may reduce thrombin mediated platelet activity Clearance by proteolysis, with minor renal contribution Short half life, no required monitoring Studied in trials which enrolled PCI pts with various comparison groups * l l Similar protection from ischemic events Superior bleeding profile compared to standard combination therapy * Replace-2, Protect TIMI 30, ACUITY, BAT TCT Presentation October 2006

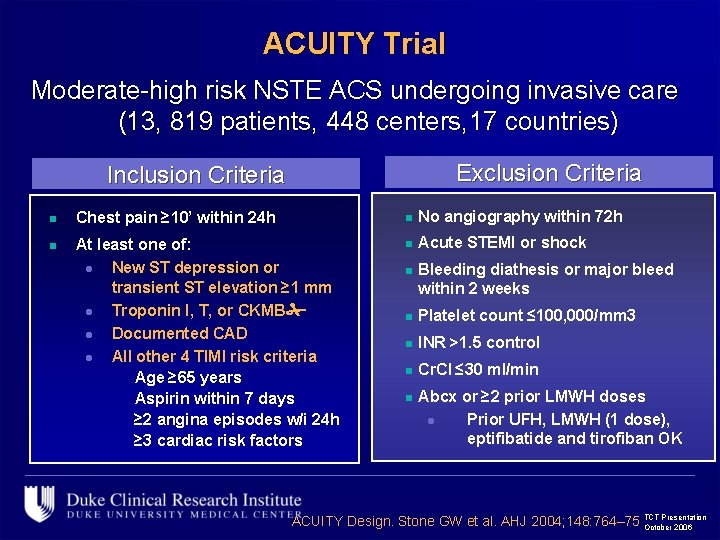
ACUITY Trial Moderate-high risk NSTE ACS undergoing invasive care (13, 819 patients, 448 centers, 17 countries) Exclusion Criteria Inclusion Criteria n Chest pain ≥ 10’ within 24 h n No angiography within 72 h n At least one of: l New ST depression or transient ST elevation ≥ 1 mm l Troponin I, T, or CKMB l Documented CAD l All other 4 TIMI risk criteria Age ≥ 65 years Aspirin within 7 days ≥ 2 angina episodes w/i 24 h ≥ 3 cardiac risk factors n Acute STEMI or shock n Bleeding diathesis or major bleed within 2 weeks n Platelet count ≤ 100, 000/mm 3 n INR >1. 5 control n Cr. Cl ≤ 30 ml/min n Abcx or ≥ 2 prior LMWH doses l Prior UFH, LMWH (1 dose), eptifibatide and tirofiban OK Presentation ACUITY Design. Stone GW et al. AHJ 2004; 148: 764– 75 TCT October 2006

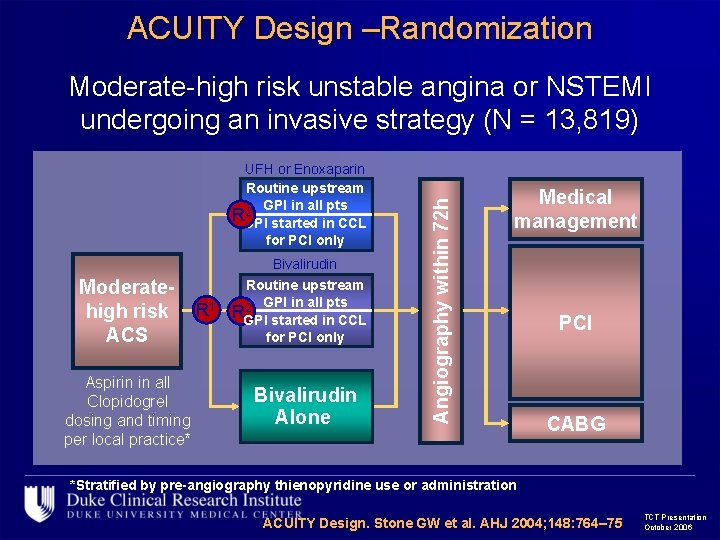
ACUITY Design –Randomization UFH or Enoxaparin Routine upstream 2 GPI in all pts RGPI started in CCL for PCI only Moderatehigh risk ACS Aspirin in all Clopidogrel dosing and timing per local practice* R 1 Bivalirudin Routine upstream 2 GPI in all pts RGPI started in CCL for PCI only Bivalirudin Alone Angiography within 72 h Moderate-high risk unstable angina or NSTEMI undergoing an invasive strategy (N = 13, 819) Medical management PCI CABG *Stratified by pre-angiography thienopyridine use or administration ACUITY Design. Stone GW et al. AHJ 2004; 148: 764– 75 TCT Presentation October 2006

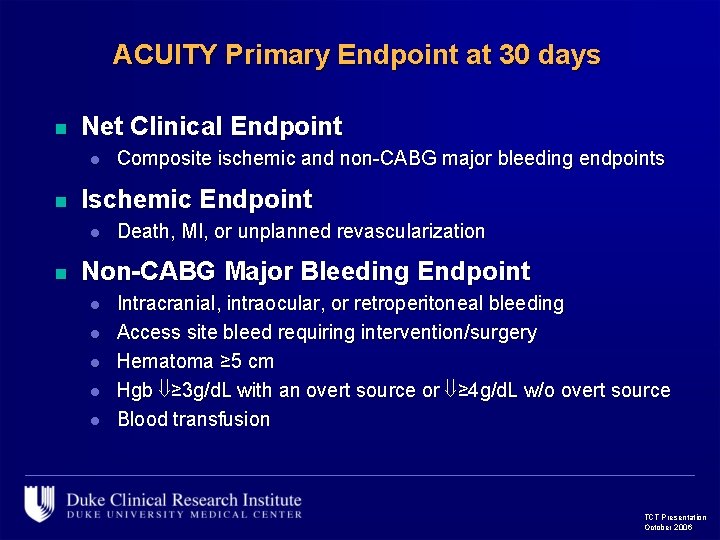
ACUITY Primary Endpoint at 30 days n Net Clinical Endpoint l n Ischemic Endpoint l n Composite ischemic and non-CABG major bleeding endpoints Death, MI, or unplanned revascularization Non-CABG Major Bleeding Endpoint l l l Intracranial, intraocular, or retroperitoneal bleeding Access site bleed requiring intervention/surgery Hematoma ≥ 5 cm Hgb ≥ 3 g/d. L with an overt source or ≥ 4 g/d. L w/o overt source Blood transfusion TCT Presentation October 2006

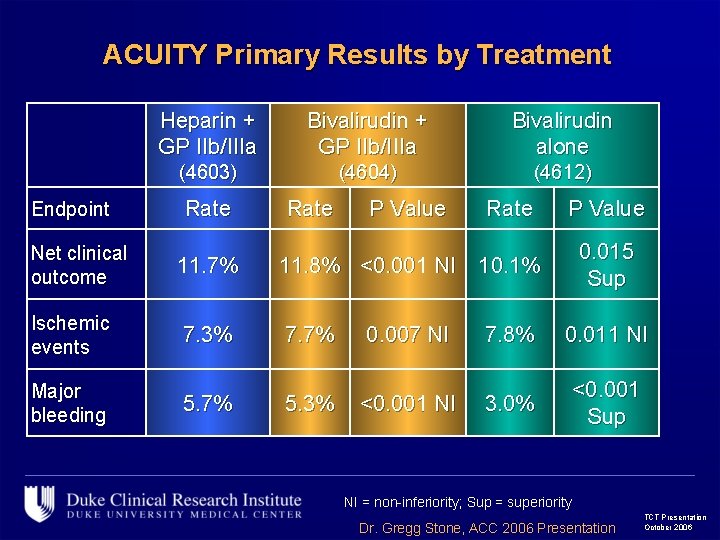
ACUITY Primary Results by Treatment Endpoint Net clinical outcome Heparin + GP IIb/IIIa Bivalirudin alone (4603) (4604) (4612) Rate P Value 0. 015 Sup 11. 7% 11. 8% <0. 001 NI 10. 1% Ischemic events 7. 3% 7. 7% 0. 007 NI 7. 8% 0. 011 NI Major bleeding 5. 7% 5. 3% <0. 001 NI 3. 0% <0. 001 Sup NI = non-inferiority; Sup = superiority Dr. Gregg Stone, ACC 2006 Presentation TCT Presentation October 2006

Purpose n To compare age subgroup results with Bivalirudin monotherapy, heparin/GPI and Bival/GPI in PCI patients in ACUITY l Ischemic Endpoints l Major and Minor Bleeding n Describe differences across age l In terms of absolute risk reduction l Among those with preserved renal function TCT Presentation October 2006

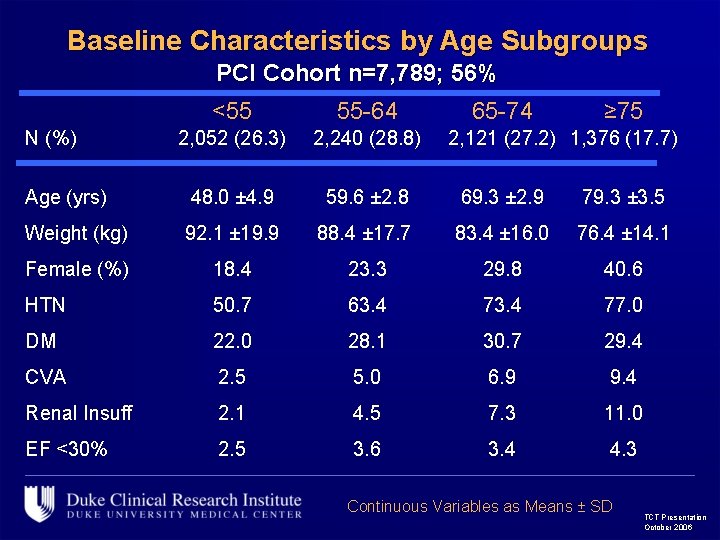
Baseline Characteristics by Age Subgroups PCI Cohort n=7, 789; 56% <55 55 -64 2, 052 (26. 3) 2, 240 (28. 8) Age (yrs) 48. 0 ± 4. 9 59. 6 ± 2. 8 69. 3 ± 2. 9 79. 3 ± 3. 5 Weight (kg) 92. 1 ± 19. 9 88. 4 ± 17. 7 83. 4 ± 16. 0 76. 4 ± 14. 1 Female (%) 18. 4 23. 3 29. 8 40. 6 HTN 50. 7 63. 4 77. 0 DM 22. 0 28. 1 30. 7 29. 4 CVA 2. 5 5. 0 6. 9 9. 4 Renal Insuff 2. 1 4. 5 7. 3 11. 0 EF <30% 2. 5 3. 6 3. 4 4. 3 N (%) 65 -74 ≥ 75 2, 121 (27. 2) 1, 376 (17. 7) Continuous Variables as Means ± SD TCT Presentation October 2006

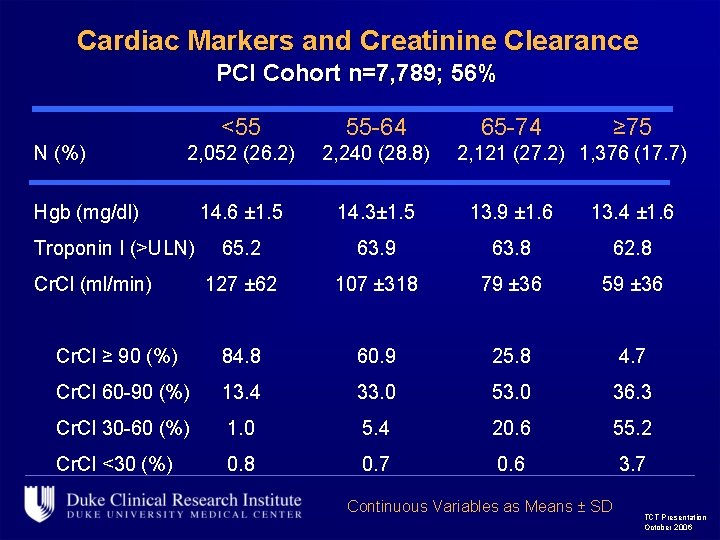
Cardiac Markers and Creatinine Clearance PCI Cohort n=7, 789; 56% <55 55 -64 2, 052 (26. 2) 2, 240 (28. 8) 14. 6 ± 1. 5 14. 3± 1. 5 13. 9 ± 1. 6 13. 4 ± 1. 6 65. 2 63. 9 63. 8 62. 8 127 ± 62 107 ± 318 79 ± 36 59 ± 36 Cr. Cl ≥ 90 (%) 84. 8 60. 9 25. 8 4. 7 Cr. Cl 60 -90 (%) 13. 4 33. 0 53. 0 36. 3 Cr. Cl 30 -60 (%) 1. 0 5. 4 20. 6 55. 2 Cr. Cl <30 (%) 0. 8 0. 7 0. 6 3. 7 N (%) Hgb (mg/dl) Troponin I (>ULN) Cr. Cl (ml/min) 65 -74 ≥ 75 2, 121 (27. 2) 1, 376 (17. 7) Continuous Variables as Means ± SD TCT Presentation October 2006

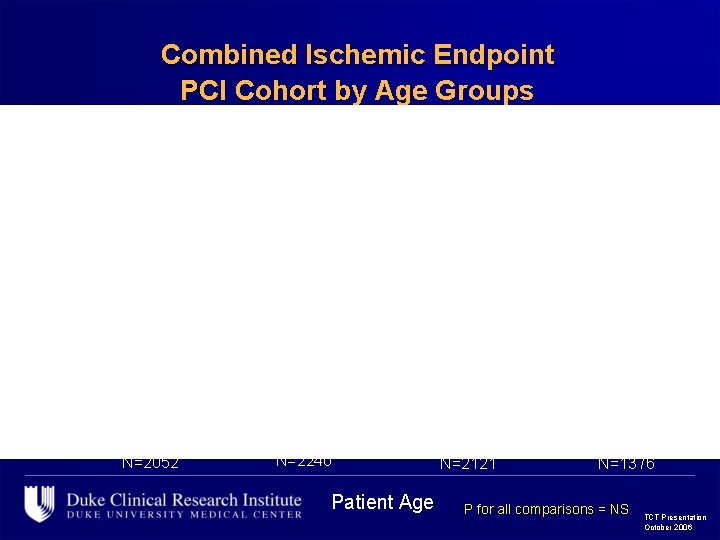
Combined Ischemic Endpoint PCI Cohort by Age Groups 12. 3 12. 2 11. 0 9. 3 8. 6 8. 3 6. 5 7. 0 N=2052 9. 0 8. 2 8. 6 7. 1 N=2240 Patient Age N=2121 N=1376 P for all comparisons = NS TCT Presentation October 2006

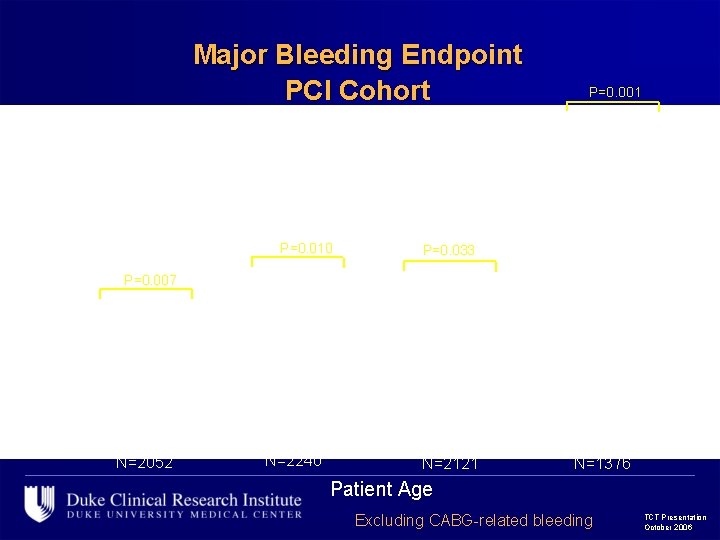
Major Bleeding Endpoint PCI Cohort P=0. 001 P<0. 001 16. 5 P=0. 010 P=0. 007 P=0. 033 P=0. 001 P=NS 6. 7 6. 6 P=0. 006 12. 3 6. 1 5. 5 5. 7 4. 2 4. 3 4. 2 3. 0 1. 7 N=2052 N=2240 N=2121 N=1376 Patient Age Excluding CABG-related bleeding TCT Presentation October 2006

Implication for Number Needed to Treat (NNT) Given the Absolute Risk Reduction (ARR) in Major Bleeding with Bivalirudin vs. Heparin/GPI 38 37 40 16 Patient Age TCT Presentation October 2006

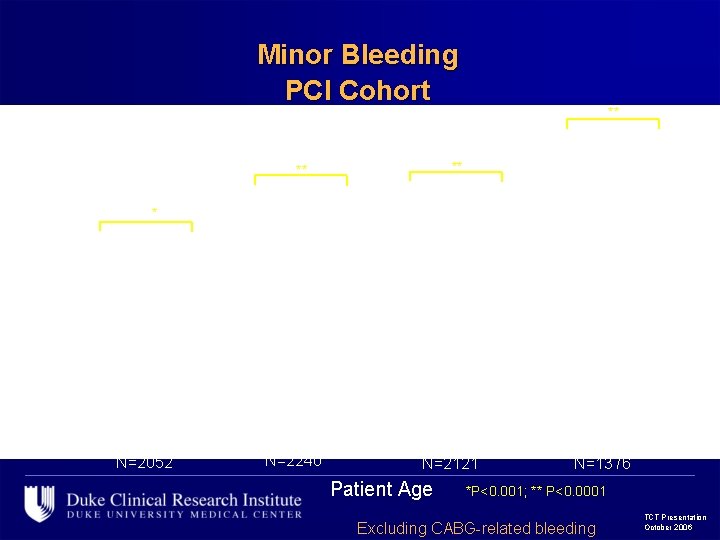
Minor Bleeding PCI Cohort ** ** ** 28. 9 ** 33. 2 35. 5 28. 6 28. 8 24. 7 22. 5 20. 6 19. 5 12. 5 N=2052 14. 3 N=2240 14. 4 N=2121 Patient Age N=1376 *P<0. 001; ** P<0. 0001 Excluding CABG-related bleeding TCT Presentation October 2006

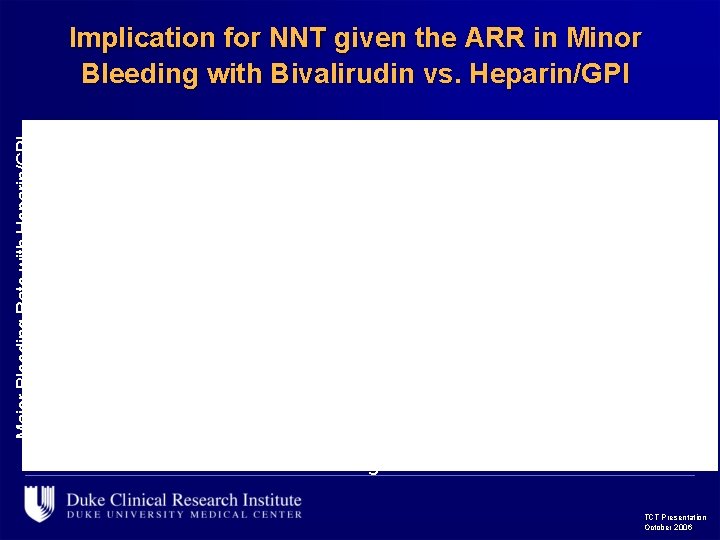
Major Bleeding Rate with Heparin/GPI Implication for NNT given the ARR in Minor Bleeding with Bivalirudin vs. Heparin/GPI 14 10 7 8 Patient Age TCT Presentation October 2006

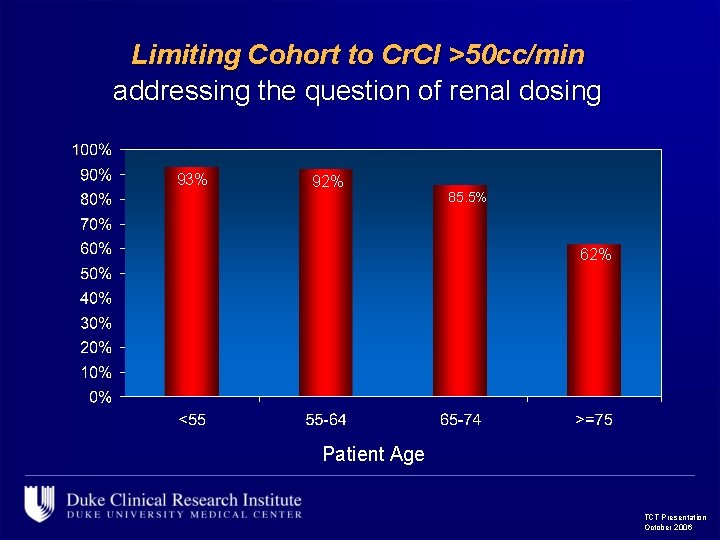
Limiting Cohort to Cr. Cl >50 cc/min addressing the question of renal dosing 93% 92% 85. 5% 62% Patient Age TCT Presentation October 2006

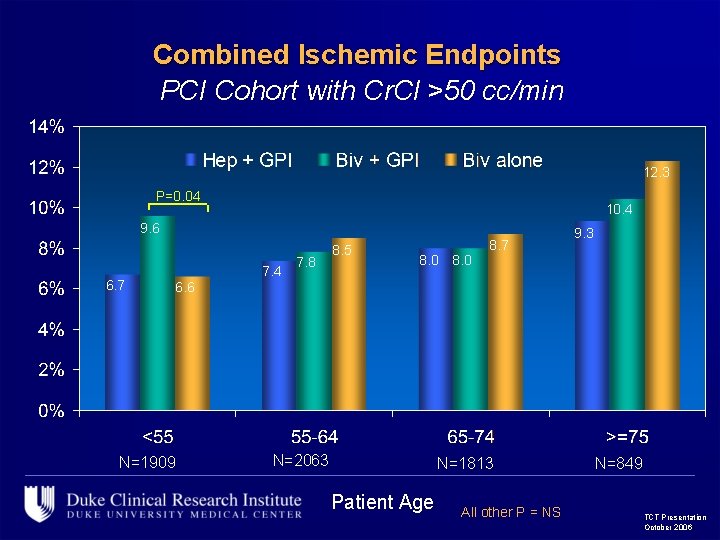
Combined Ischemic Endpoints PCI Cohort with Cr. Cl >50 cc/min 12. 3 P=0. 04 10. 4 9. 6 6. 7 7. 4 7. 8 8. 5 8. 0 8. 7 9. 3 6. 6 N=1909 N=2063 N=1813 Patient Age All other P = NS N=849 TCT Presentation October 2006

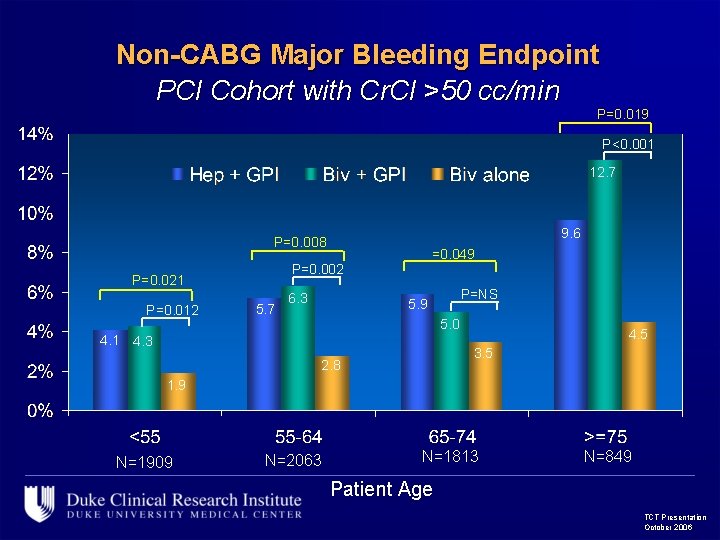
Non-CABG Major Bleeding Endpoint PCI Cohort with Cr. Cl >50 cc/min P=0. 019 P<0. 001 12. 7 9. 6 P=0. 008 P=0. 002 P=0. 021 P=0. 012 =0. 049 5. 7 6. 3 P=NS 5. 9 5. 0 4. 1 4. 3 4. 5 3. 5 2. 8 1. 9 N=1909 N=2063 N=1813 N=849 Patient Age TCT Presentation October 2006

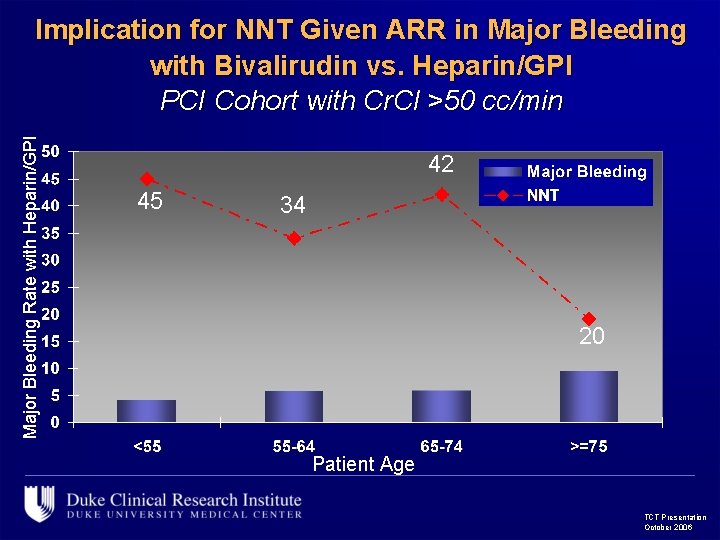
Major Bleeding Rate with Heparin/GPI Implication for NNT Given ARR in Major Bleeding with Bivalirudin vs. Heparin/GPI PCI Cohort with Cr. Cl >50 cc/min 42 45 34 20 Patient Age TCT Presentation October 2006

Conclusions n Ischemic and hemorrhagic events increase with age n Across all age groups, bivalirudin is associated with significantly less major and minor bleeding and similar ischemic outcomes l l n Even among those with preserved renal function ARR for major bleeding was greatest in the elderly (age >75) NNT of 16 to prevent one major bleed NNT of 8 to prevent one minor bleed Dose all agents carefully, fewer agents may be better TCT Presentation October 2006