Outcome evaluation of screening WeiChu Chie outcome researchscreening

























- Slides: 40

Outcome evaluation of screening Wei-Chu Chie outcome research/screening 1

Screening • Secondary prevention – early detection and prompt treatment – screening people with early, often preclinical, manifestations of diseases, to prevent its progression – must followed by diagnosis and treatment procedures outcome research/screening 2

Variants • Mass screening • opportunistic screening • clinical case finding outcome research/screening 3

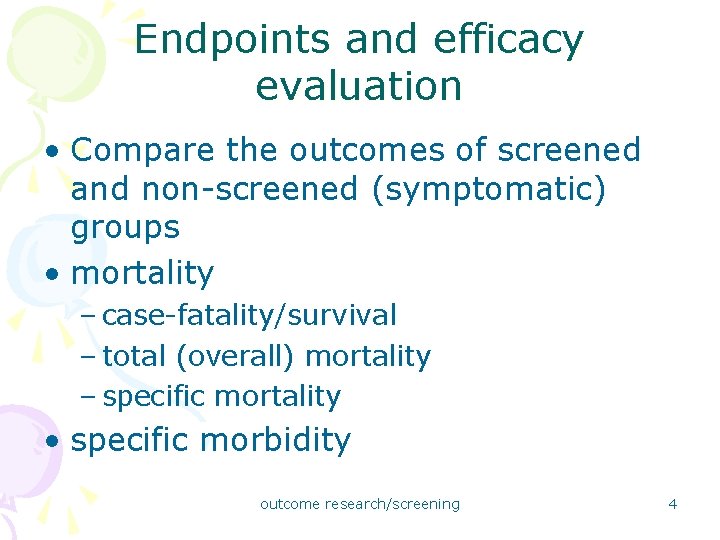
Endpoints and efficacy evaluation • Compare the outcomes of screened and non-screened (symptomatic) groups • mortality – case-fatality/survival – total (overall) mortality – specific mortality • specific morbidity outcome research/screening 4

Case-fatality/survival • Count from the time of diagnosis • take only causes of death that related to the target disease(s) of screening – lead-time bias – length bias (prognosis selection bias) outcome research/screening 5

Overall mortality • Count from the time of participation • take all causes of death – unaffected by the ways of diagnosis or detection – overall impact of the program • positive & negative – sometimes insensitive outcome research/screening 6

Specific mortality • Count from the time of participation • take only causes of death that related to the target disease(s) of screening – may be affected by the ways of diagnosis or detection – more sensitive – sometimes difficult to identify outcome research/screening 7

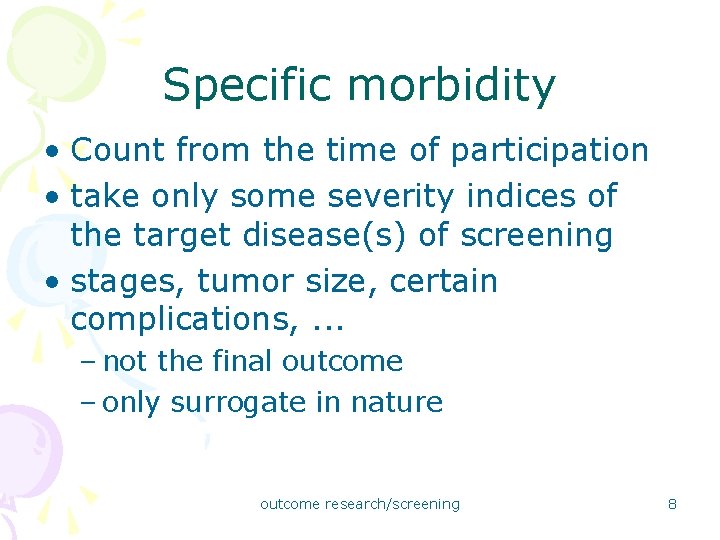
Specific morbidity • Count from the time of participation • take only some severity indices of the target disease(s) of screening • stages, tumor size, certain complications, . . . – not the final outcome – only surrogate in nature outcome research/screening 8

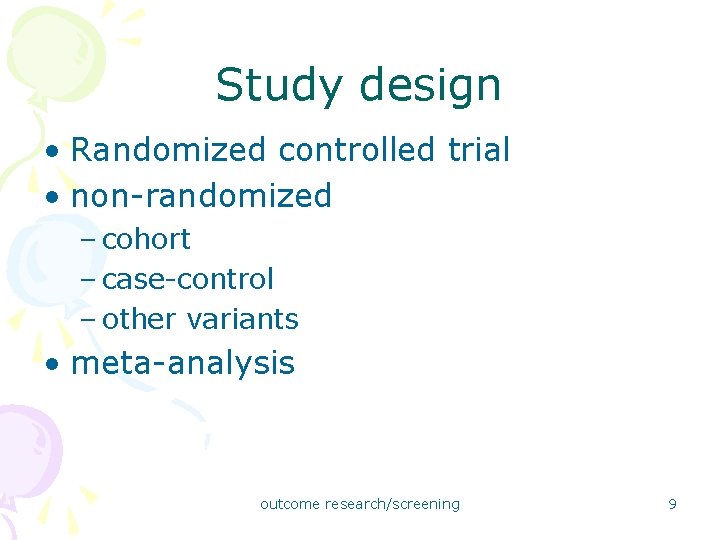
Study design • Randomized controlled trial • non-randomized – cohort – case-control – other variants • meta-analysis outcome research/screening 9

實驗性方法的三要素 • 實驗單位 (experimental unit) • 處置 (treatment) • 評估 (evaluation) outcome research/screening 10




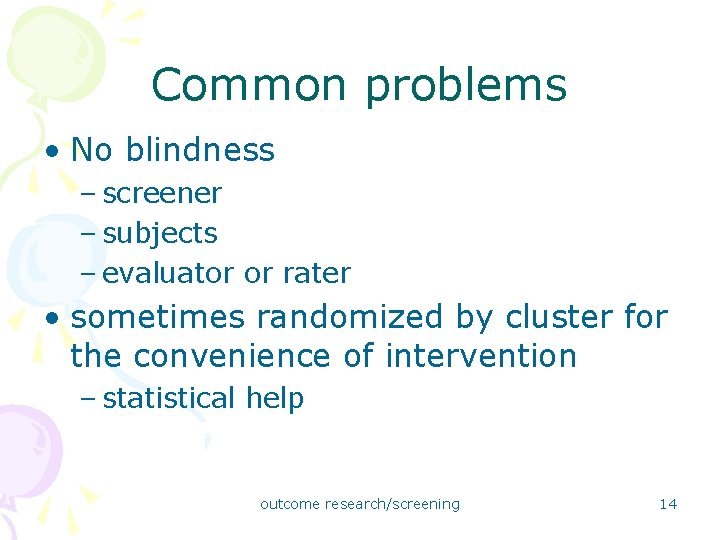
Common problems • No blindness – screener – subjects – evaluator or rater • sometimes randomized by cluster for the convenience of intervention – statistical help outcome research/screening 14

Examples • Breast self-examination for breast cancer – mass screening • General health screening for cardiovascular risks – by general practitioners • Case-finding for depression in primary care – by general practitioners outcome research/screening 15

BSE • Thomas DB. , et al. Randomized trial of breast self-examination in Shanghai: final results. JNCI 2002; 94: 1445 -1457. outcome research/screening 16

BSE: background/goal/hypothesis • Background: – BSE efficacy unproven • Goal: – to determine whether. . . • Hypothesis: – an intensive program of BSE instruction will reduce the mortality (number of death ) of breast cancer outcome research/screening 17

BSE: study design • Randomized controlled trial – instruction group – control group – randomized by cluster (factories) stratified by hospital affiliation – no statistical adjustment: large number and identical rates • mass screening outcome research/screening 18

BSE: subjects • Shanghai Textile Industry Bureau (STIB) employees • women born between 1925 -1958 (31 -64) • eligibility, inclusion/exclusion criteria • 289292 …. 266064 – instruction: 146437 …. 132979 – control: 142955 …. 133085 outcome research/screening 19

BSE: exposure/intervention • BSE instruction – small group (10) – video/practice/discussion/reinforcement – review/supervision – proficiency assessment outcome research/screening 20

BSE: endpoint • Primary: death from breast cancer – definition – judgement: blind • Secondary? – Intermediate variables: benign tumors/biopsies • Follow-up: 11 years outcome research/screening 21

BSE: data analysis • Survival – from time of entry – from time of diagnosis • Kaplan-Meier/log-rank test • Cox proportional hazards model • comparability , compliance, … • intermediate variables outcome research/screening 22

BSE: major results/discussion • Mortality/survival – from entry – from diagnosis • comparability, compliance, … • intermediate variables – benign tumors … biopsy (unnecessary burden) outcome research/screening 23

CRS • Engberg M. , et al. General health screenings to improve cardiovascular risk profiles: a randomized controlled trial in general practice with 5 -year follow-up. J Family Practice 2002; 51: 546 -552. outcome research/screening 24

CRS: background/goal/hypothesis • Background: Danish trial – GP’s role in primary prevention for CVD • Goal: – to investigate the impact of general health screenings and health discussions on CV risk profiles • Hypothesis: – … can reduce. . . outcome research/screening 25

CRS: study design • Randomized controlled trial – 3 groups – by individual – stratified randomization based on • GP, sex, age, cohabitation status, BMI – too many cells • GP X sex X age X … – stratified factors should be treated as covariates outcome research/screening 26

Stratified randomization – Individuals stratified by certain characteristics – Independent random assignment to different groups in each cell • eg. Old-male, old-female, young-male, … • random code for each consecutive subject • can be generated by computer – To avoid imbalance • may have empty cells if N is small • no need when N is large outcome research/screening 27

CRS: subjects • Ebeltoft, Aarhus County, Denmark • 9 GPs (general practitioners) • 3464 … random sample 2000 inhabitants • 30 -49 years outcome research/screening 28

CRS: exposure/intervention • Health screening & follow-up discussion – Group 1: health screening (502) – Group 2: health screening+follow-up discussion (504) – Group 3: none (control) (501) – All three: questionnaire outcome research/screening 29

CRS: endpoint • Primary: score and categorical (high/low) – CRS: gender, family inheritance (CAD <55 y), tobacco use, BP, total serum cholesterol, BMI – BMI, SBP, DBP, serum cholesterol • by smoking and overweight subgroup analysis – No blindness • Follow-up: 5 years outcome research/screening 30

CRS: data analysis • T-test, chi-square, … • intention-to-treat • compare values or high score rates instead of changes from baseline – endpoints – baseline data – follow-up rates outcome research/screening 31

CRS: major results/discussion • CRS, BMI, and serum cholesterol levels were lower in the Intervention group • subgroup analysis: high vs. low risk ones • no differences between two intervention groups • not followed to the final endpoint outcome research/screening 32

Depression • Williams JW. , et al. Case-finding for depression in primary care: a randomized trial. Am J Med 1999; 106: 36 -43. outcome research/screening 33

Dep: background/goal/hypothesis • Background: – impact of depression/role of primary care • Goal: – evaluate the efficacy of two case-finding instruments vs. usual care • Hypothesis: – … can enhance symptom reduction/recovery for depression patients outcome research/screening 34

Dep: study design • Randomized controlled trial – 3 groups – by individual – random assignment stratified by site • computer-generated randomization blocked log outcome research/screening 35

Dep: subjects – GP and internal medicine clinics in San Antonio, Texas and Washington, DC. – Consecutive adult patients – Only those diagnosed as with depression were analyzed • screened with case-finding: positive--additional questioning for clinical depression • all patients: DSM-III-R, CAGE, SF-36, satisfaction • rater (evaluator) blindness outcome research/screening 36

Dep: exposure/intervention • Case-finding instruments screening – Group 1: single question (330) – Group 2: 20 item questionnaire (323) – Group 3: usual care (control) (316) outcome research/screening 37

Dep: endpoint • Primary: only depressive patients – recovery rates (<=1 DSM-III-R symptoms) – symptoms score changes from baseline • Secondary: – satisfaction • Follow-up: 3 months outcome research/screening 38

Dep: data analysis • Proportion and rates • Symptom numbers – only for patients diagnosed as with depressions – not severity indices – by medical record: counseling, referral, medicine • baseline data • follow-up rates outcome research/screening 39

Dep: major results/discussion • Recovery rate: – case-finding better than usual care • symptoms numbers – not different • Only diagnosed patients were analyzed • not followed to the final endpoint outcome research/screening 40
 Which reaction does akira most fear from chie?
Which reaction does akira most fear from chie? Counseling outcome research and evaluation
Counseling outcome research and evaluation Outcome evaluation
Outcome evaluation Outcome based evaluation
Outcome based evaluation Fica spiritual assessment tool
Fica spiritual assessment tool Preimplantation genetic screening pros and cons
Preimplantation genetic screening pros and cons Iceberg phenomenon of disease
Iceberg phenomenon of disease Zeff of potassium
Zeff of potassium Product-market screening criteria should
Product-market screening criteria should Expanded newborn screening program
Expanded newborn screening program Mass_947
Mass_947 Bolton breast screening
Bolton breast screening Harmful use
Harmful use Simple screening instrument for substance abuse
Simple screening instrument for substance abuse Definitive screening design
Definitive screening design Discounted payback formula
Discounted payback formula Mobile occupational health screening unit
Mobile occupational health screening unit Market segmentation matrix
Market segmentation matrix Use case diagram for restaurant finder
Use case diagram for restaurant finder Screening session
Screening session Down syndrome screening results
Down syndrome screening results Dyscalculia screening test
Dyscalculia screening test Shallow screening of ballast
Shallow screening of ballast Where to do a heel stick on newborn
Where to do a heel stick on newborn Idea screening
Idea screening Surp-p screening tool
Surp-p screening tool Import javax.swing
Import javax.swing Zoho background checks
Zoho background checks Coa statement example
Coa statement example Screening and selecting employees international
Screening and selecting employees international Magister project management
Magister project management Idea generation contoh
Idea generation contoh Paralleltestmethode
Paralleltestmethode Tscc scoring sheet
Tscc scoring sheet Clearinghouse applicant initiated
Clearinghouse applicant initiated Tsa certified cargo screening program
Tsa certified cargo screening program Peehip wellness
Peehip wellness Business partner screening
Business partner screening Class diagram for airport check-in and security screening
Class diagram for airport check-in and security screening Society for biomolecular screening
Society for biomolecular screening Hologic's low dose 3d mammography
Hologic's low dose 3d mammography