Osmotic Fragility Lab Unit I Problem 2 Homeostasis

Osmotic Fragility Lab Unit I Problem 2 Homeostasis A 12/14/2021 1
Objectives 1. To define osmosis , osmotic pressure 2. To understand the terms: osmolarity, osmolality and tonicity 3. To know how to calculate the osmolarity of the plasma 4. To be able to calculate the osmolarity of different Na. Cl Solution 5. To learn the significance and practice the measurement of osmotic fragility of red blood cells 12/14/2021 2
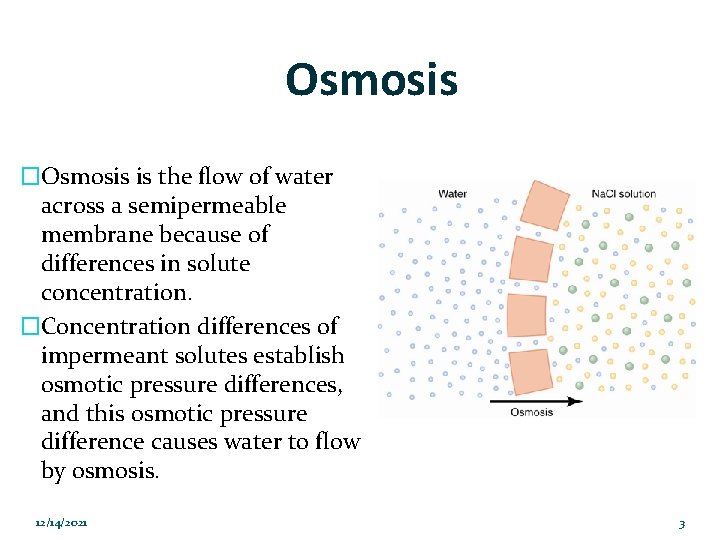
Osmosis �Osmosis is the flow of water across a semipermeable membrane because of differences in solute concentration. �Concentration differences of impermeant solutes establish osmotic pressure differences, and this osmotic pressure difference causes water to flow by osmosis. 12/14/2021 3
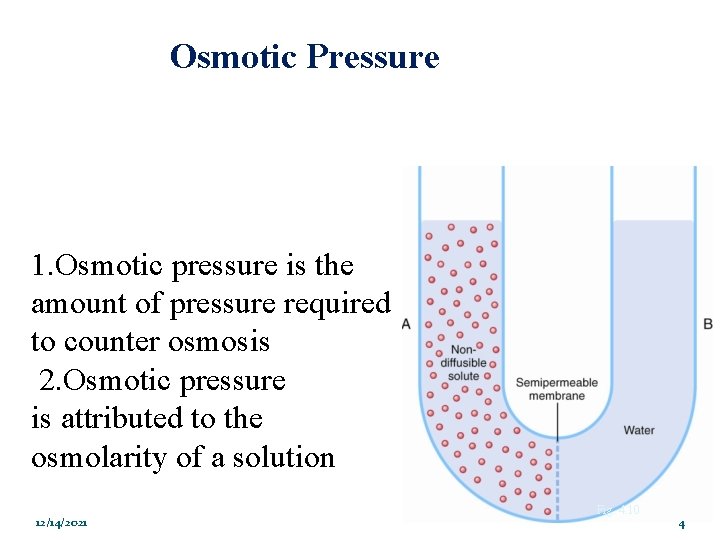
Osmotic Pressure: 1. Osmotic pressure is the amount of pressure required to counter osmosis 2. Osmotic pressure is attributed to the osmolarity of a solution 12/14/2021 Fig. 4. 10 4
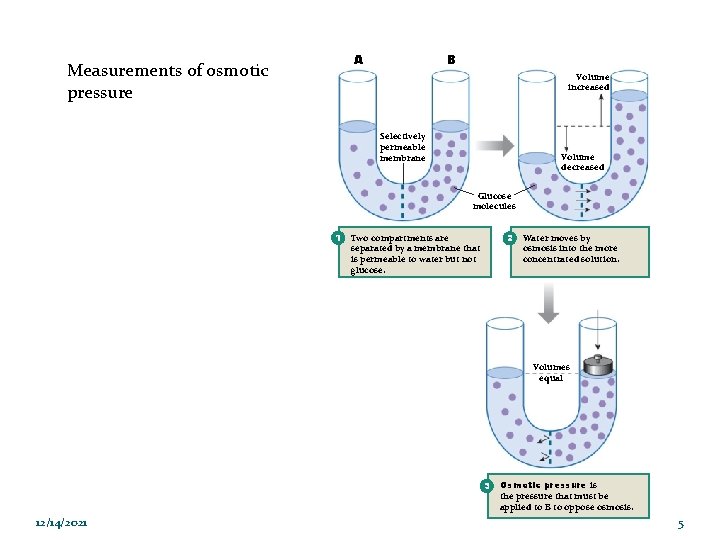
A Measurements of osmotic pressure B Volume increased Selectively permeable membrane Volume decreased Glucose molecules 1 2 Two compartments are separated by a membrane that is permeable to water but not glucose. Water moves by osmosis into the more concentrated solution. Volumes equal 3 12/14/2021 Osmotic pressure is the pressure that must be applied to B to oppose osmosis. 5
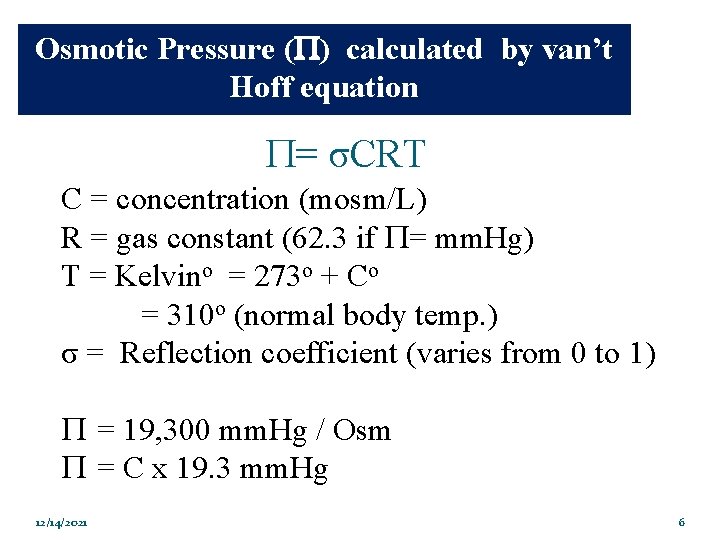
Osmotic Pressure ( ) calculated by van’t Hoff equation P= σCRT C = concentration (mosm/L) R = gas constant (62. 3 if P= mm. Hg) T = Kelvino = 273 o + Co = 310 o (normal body temp. ) σ = Reflection coefficient (varies from 0 to 1) P = 19, 300 mm. Hg / Osm P = C x 19. 3 mm. Hg 12/14/2021 6
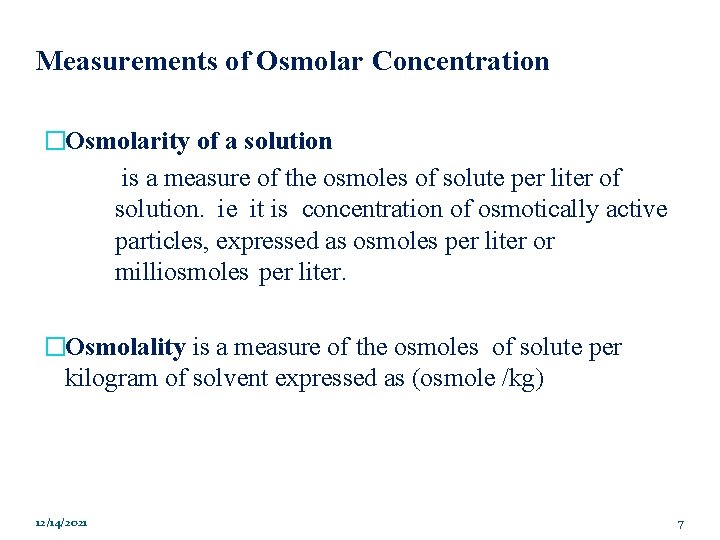
Measurements of Osmolar Concentration �Osmolarity of a solution is a measure of the osmoles of solute per liter of solution. ie it is concentration of osmotically active particles, expressed as osmoles per liter or milliosmoles per liter. �Osmolality is a measure of the osmoles of solute per kilogram of solvent expressed as (osmole /kg) 12/14/2021 7
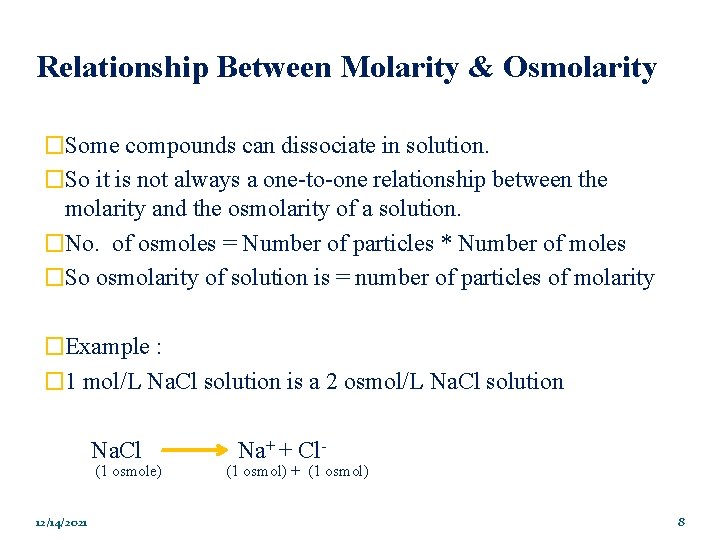
Relationship Between Molarity & Osmolarity �Some compounds can dissociate in solution. �So it is not always a one-to-one relationship between the molarity and the osmolarity of a solution. �No. of osmoles = Number of particles * Number of moles �So osmolarity of solution is = number of particles of molarity �Example : � 1 mol/L Na. Cl solution is a 2 osmol/L Na. Cl solution Na. Cl (1 osmole) 12/14/2021 Na+ + Cl- (1 osmol) + (1 osmol) 8
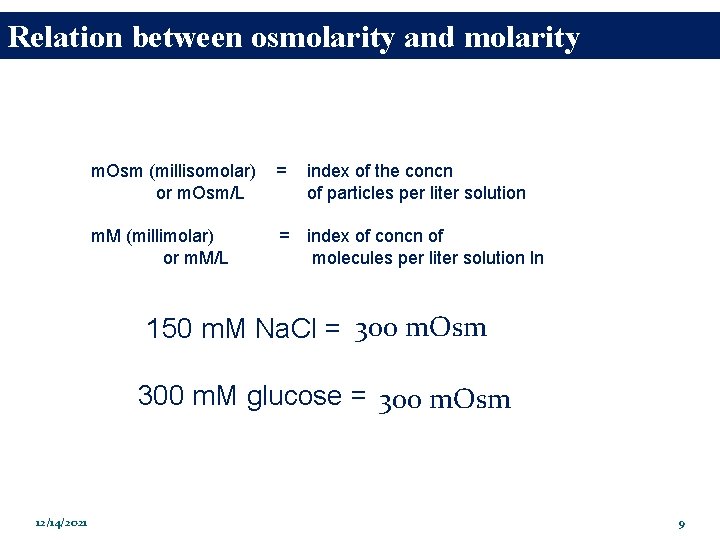
Relation between osmolarity and molarity m. Osm (millisomolar) or m. Osm/L = index of the concn of particles per liter solution m. M (millimolar) or m. M/L = index of concn of molecules per liter solution ln 150 m. M Na. Cl = 300 m. Osm 300 m. M glucose = 300 m. Osm 12/14/2021 9
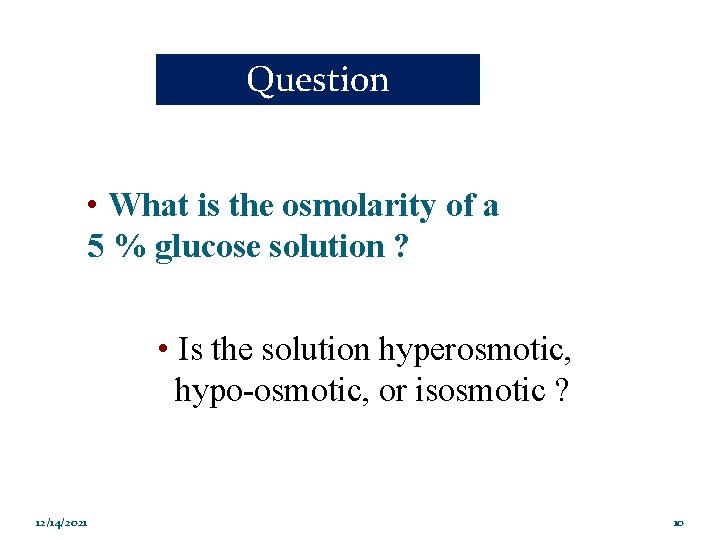
Question • What is the osmolarity of a 5 % glucose solution ? • Is the solution hyperosmotic, hypo-osmotic, or isosmotic ? 12/14/2021 10
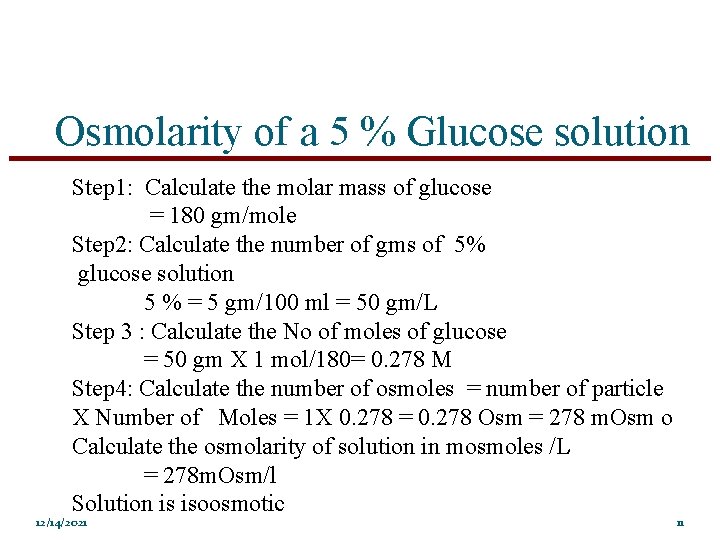
Osmolarity of a 5 % Glucose solution Step 1: Calculate the molar mass of glucose = 180 gm/mole Step 2: Calculate the number of gms of 5% glucose solution 5 % = 5 gm/100 ml = 50 gm/L Step 3 : Calculate the No of moles of glucose = 50 gm X 1 mol/180= 0. 278 M Step 4: Calculate the number of osmoles = number of particle X Number of Moles = 1 X 0. 278 = 0. 278 Osm = 278 m. Osm o Calculate the osmolarity of solution in mosmoles /L = 278 m. Osm/l Solution is isoosmotic 12/14/2021 11

Question • What is the osmolarity of a 3. 0 % Na. Cl solution ? • Is the solution hypertonic, hypotonic, or isotonic ? 12/14/2021 12
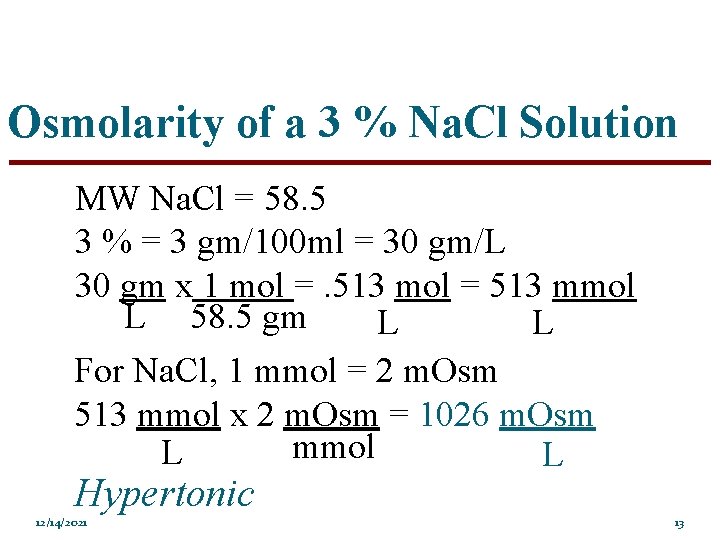
Osmolarity of a 3 % Na. Cl Solution MW Na. Cl = 58. 5 3 % = 3 gm/100 ml = 30 gm/L 30 gm x 1 mol =. 513 mol = 513 mmol L 58. 5 gm L L For Na. Cl, 1 mmol = 2 m. Osm 513 mmol x 2 m. Osm = 1026 m. Osm mmol L L Hypertonic 12/14/2021 13

Terminology Isosmotic - has same osmolarity as body fluids Hyperosmotic - higher osmolarity than body fluids Hyposmotic- lower osmolarity than body fluids 12/14/2021 14
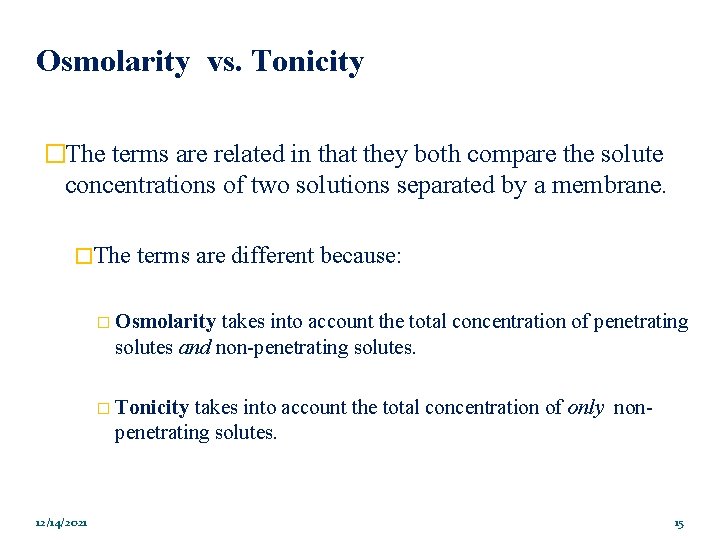
Osmolarity vs. Tonicity �The terms are related in that they both compare the solute concentrations of two solutions separated by a membrane. �The terms are different because: � Osmolarity takes into account the total concentration of penetrating solutes and non-penetrating solutes. � Tonicity takes into account the total concentration of only nonpenetrating solutes. 12/14/2021 15
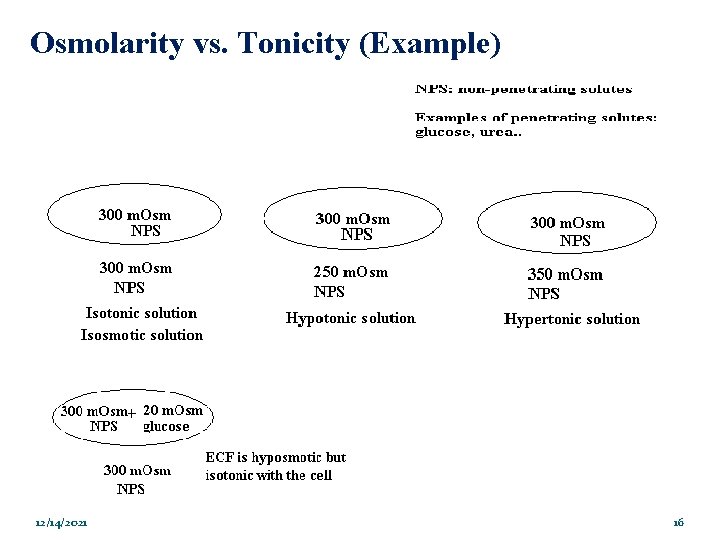
Osmolarity vs. Tonicity (Example) 12/14/2021 16
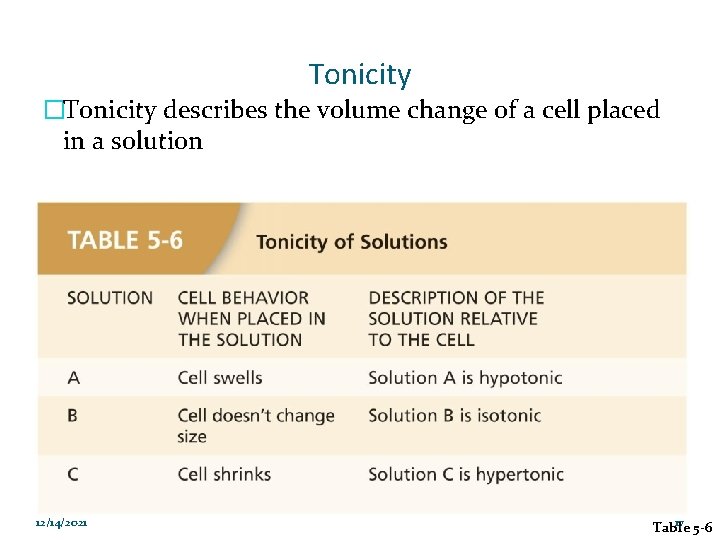
Tonicity �Tonicity describes the volume change of a cell placed in a solution 12/14/2021 17 Table 5 -6
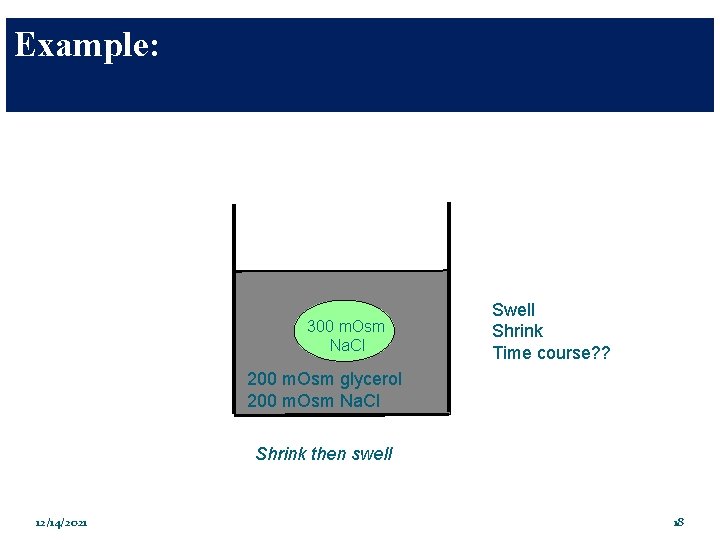
Example: 300 m. Osm Na. Cl Swell Shrink Time course? ? 200 m. Osm glycerol 200 m. Osm Na. Cl Shrink then swell 12/14/2021 18
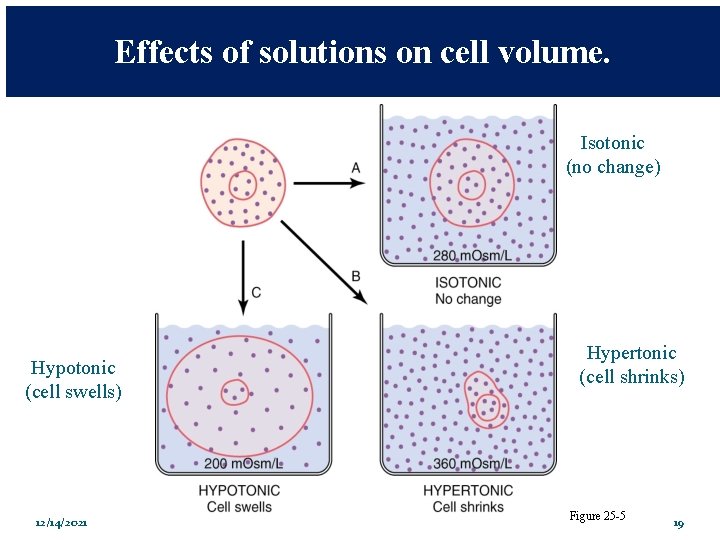
Effects of solutions on cell volume. Isotonic (no change) Hypotonic (cell swells) 12/14/2021 Hypertonic (cell shrinks) Figure 25 -5 19
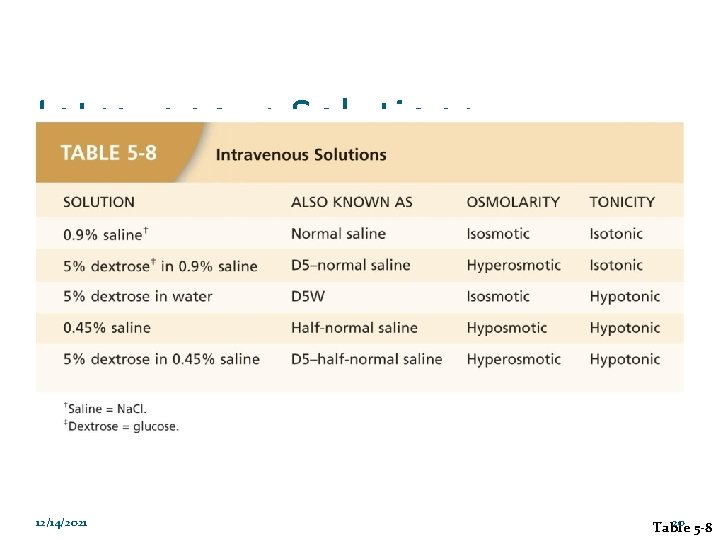
Intravenous Solutions 12/14/2021 20 Table 5 -8
![Estimating Plasma Osmolarity � Dominated by [Na+] and associated anions � Under normal conditions, Estimating Plasma Osmolarity � Dominated by [Na+] and associated anions � Under normal conditions,](http://slidetodoc.com/presentation_image_h2/0a49e7242a57449e67af542bd37ee7f3/image-21.jpg)
Estimating Plasma Osmolarity � Dominated by [Na+] and associated anions � Under normal conditions, ECF osmolarity can be roughly estimated as: POSM = 2 [Na+]p = 270 -290 m. Osm �Normal plasma osmolarity = 2[Na] + [glucose] + [BUN] 12/14/2021 21
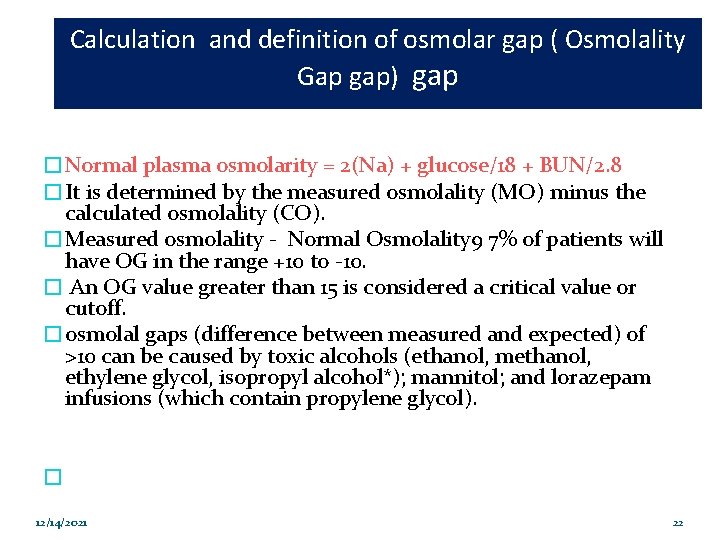
Calculation and definition of osmolar gap ( Osmolality Gap gap) gap �Normal plasma osmolarity = 2(Na) + glucose/18 + BUN/2. 8 �It is determined by the measured osmolality (MO) minus the calculated osmolality (CO). �Measured osmolality - Normal Osmolality 9 7% of patients will have OG in the range +10 to -10. � An OG value greater than 15 is considered a critical value or cutoff. �osmolal gaps (difference between measured and expected) of >10 can be caused by toxic alcohols (ethanol, methanol, ethylene glycol, isopropyl alcohol*); mannitol; and lorazepam infusions (which contain propylene glycol). � 12/14/2021 22
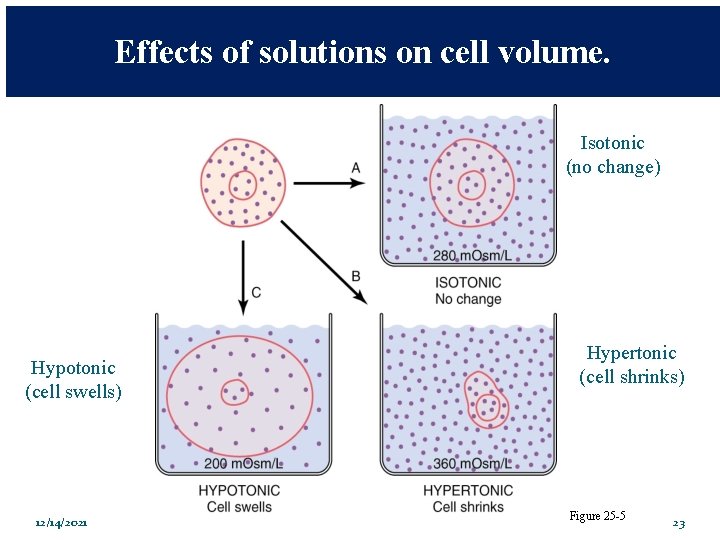
Effects of solutions on cell volume. Isotonic (no change) Hypotonic (cell swells) 12/14/2021 Hypertonic (cell shrinks) Figure 25 -5 23
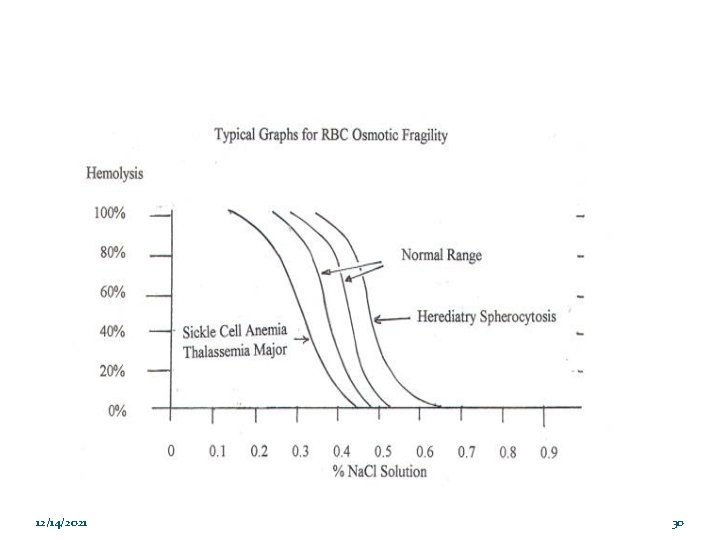
Osmotic Fragility Test � Osmotic fragility is a test to measures red blood cell (RBC) resistance to hemolysis when exposed to a series of increasingly diluted saline (hypotonic) solutions. � The sooner hemolysis occurs, the greater the osmotic fragility of the cells. � Osmotic fragility test often performed to aid with diagnosis of diseases associated with RBC membrane abnormalities. 12/14/2021 24

Osmotic Fragility Test Osmotic fragility most often expressed in terms of the highest concentration of saline at which lysis is just detectable (initial lysis or minimum resistance) and the highest concentration of saline in which lysis appears to be complete (complete lysis or maximum resistance). Normal Hemolysis: 12/14/2021 Starts in 0. 45% Na. Cl solution Completes in 0. 35% - 0. 30% Na. Cl solution 25
Factors that Affect Osmotic Fragility 1. Cell membrane composition and integrity Surface-to- Volume ratio : The larger the amount of red cell membrane (surface area) in relation to the size of the cell, the more rupturing the cell is capable of absorbing before Spherocytes have a decreased surface area-to-volume ratio; their ability to take in water before stretching the surface membrane is thus more limited than normal. 2. 12/14/2021 26
Procedure Specimen: �Heparinized venous blood �The test should be set up within 2 hours of collection, or within 6 hours if the blood is refrigerated. Equipment: �Test tubes �Measuring pipettes �Distilled water � 1% Na. Cl solution 12/14/2021 27
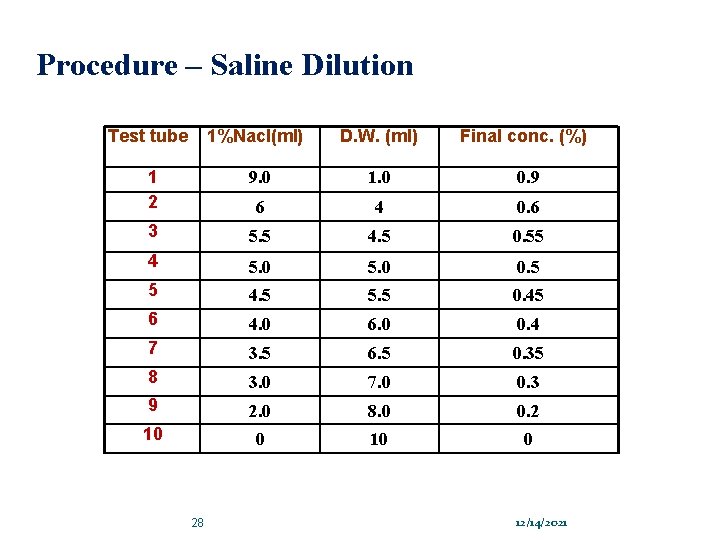
Procedure – Saline Dilution Test tube 1%Nacl(ml) D. W. (ml) Final conc. (%) 1 2 9. 0 1. 0 0. 9 6 4 0. 6 3 5. 5 4. 5 0. 55 4 5. 0 0. 5 5 4. 5 5. 5 0. 45 6 4. 0 6. 0 0. 4 7 3. 5 6. 5 0. 35 8 3. 0 7. 0 0. 3 9 2. 0 8. 0 0. 2 10 0 28 12/14/2021
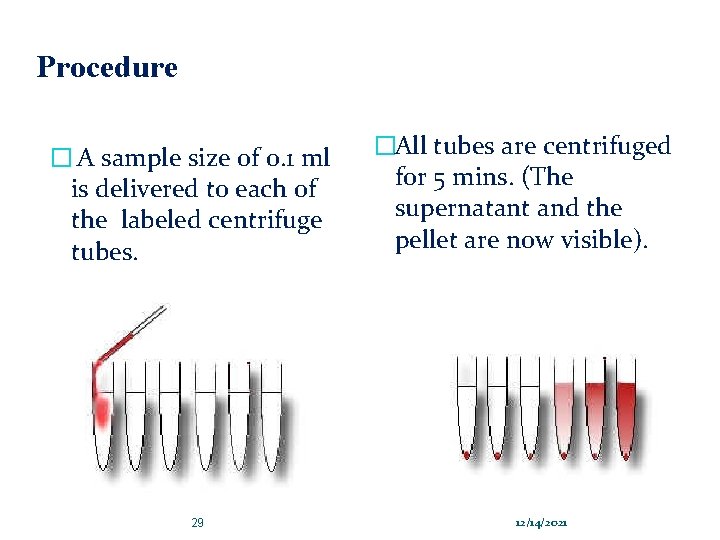
Procedure � A sample size of 0. 1 ml is delivered to each of the labeled centrifuge tubes. 29 �All tubes are centrifuged for 5 mins. (The supernatant and the pellet are now visible). 12/14/2021
12/14/2021 30
Factors Affecting Osmotic Fragility Tests 1. Presence of hemolytic organisms in the sample 2. Severe anemia or other conditions with fewer RBCs available for testing 3. Recent blood transfusion 4. Old sample (older red cells are more fragile) 12/14/2021 31
Experimental Factors Affecting Osmotic Fragility Tests �Three variables capable of markedly affecting the results must be controlled: 1. The relative volumes of blood and saline 2. The final p. H of the blood in saline suspension (a shift of 0. 1 of a p. H unit is equivalent to altering the saline concentration by 0. 1 g/l, fragility of RBCs being increased by a decrease in p. H) 3. The temperature at which the tests are carried out (An increase in temperature decreases the fragility, an increase of 5°C being equivalent to an increase in saline concentration of about 0. 1 g/l). 12/14/2021 32
- Slides: 32